Propylthiouracil - Propylthiouracil tablet prescribing information
WARNING
Severe liver injury and acute liver failure, in some cases fatal, have been reported in patients treated with propylthiouracil. These reports of hepatic reactions include cases requiring liver transplantation in adult and pediatric patients. Propylthiouracil should be reserved for patients who cannot tolerate methimazole and in whom radioactive iodine therapy or surgery are not appropriate treatments for the management of hyperthyroidism. Propylthiouracil may be the treatment of choice when an antithyroid drug is indicated during or just prior to the first trimester of pregnancy (see WARNINGS and PRECAUTIONS ).
INDICATIONS AND USAGE
Propylthiouracil tablets are indicated:
- in patients with Graves’ disease with hyperthyroidism or toxic multinodular goiter who are intolerant of methimazole and for whom surgery or radioactive iodine therapy is not an appropriate treatment option
- to ameliorate symptoms of hyperthyroidism in preparation for thyroidectomy or radioactive iodine therapy in patients who are intolerant of methimazole
DOSAGE AND ADMINISTRATION
Propylthiouracil is administered orally. The total daily dosage is usually given in 3 equal doses at approximately 8-hour intervals.
Adults
The initial dose is 300 mg daily. In patients with severe hyperthyroidism, very large goiters, or both, the initial dose may be increased to 400 mg daily; an occasional patient will require 600 to 900 mg daily initially. The usual maintenance dose is 100 to 150 mg daily.
Pediatric Patients
Propylthiouracil is generally not recommended for use in the pediatric patient population except in rare instances in which other alternative therapies are not appropriate options. Studies evaluating appropriate dosing regimen have not been conducted in the pediatric population although general practice would suggest initiation of therapy in patients 6 years or older at a dosage of 50 mg daily with careful upward titration based on clinical response and evaluation of TSH and free T4 levels. Although cases of severe liver injury have been reported with doses as low as 50 mg/day, most cases were associated with doses of 300 mg/day and higher.
Geriatric Patients
Clinical studies of propylthiouracil did not include sufficient numbers of subjects aged 65 or over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
In general, dose selection for an elderly patient should be cautious reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
CONTRAINDICATIONS
Propylthiouracil is contraindicated in patients who have demonstrated hypersensitivity to the drug or any of the other product components.
ADVERSE REACTIONS
The following adverse reactions have been reported with the use of propylthiouracil. Because these events generally come from voluntary reporting from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Severe adverse reactions include liver injury presenting as hepatitis, liver failure necessitating liver transplantation or resulting in death (see WARNINGS ). Inhibition of myelopoiesis (agranulocytosis, granulopenia, aplastic anemia, and thrombocytopenia), drug fever, a lupus-like syndrome (including splenomegaly and vasculitis), periarteritis, hypoprothrombinemia, and bleeding have been reported. Nephritis, glomerulonephritis, interstitial pneumonitis, exfoliative dermatitis, and erythema nodosum have also been reported.
There are reports of a vasculitis associated with the presence of anti-neutrophilic cytoplasmic antibodies (ANCA), resulting in severe complications and death (see WARNINGS ).
There have been rare reports of serious hypersensitivity reactions (e.g., Stevens Johnson syndrome and toxic epidermal necrolysis) in patients treated with propylthiouracil. Other adverse reactions include skin rash, uticaria, nausea, vomiting, epigastric distress, arthralgia, paresthesias, loss of taste, taste perversion, abnormal loss of hair, myalgia, headache, pruritus, drowsiness, neuritis, edema, vertigo, skin pigmentation, jaundice, sialadenopathy, and lymphadenopathy.
To report SUSPECTED ADVERSE EVENTS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch for voluntary reporting of adverse reactions.
Drug Interactions
Anticoagulants (oral): Due to the potential inhibition of vitamin K activity by propylthiouracil, the activity of oral anticoagulants (e.g., warfarin) may be increased; additional monitoring of PT/INR should be considered, especially before surgical procedures.
Beta-adrenergic blocking agents: Hyperthyroidism may cause an increased clearance of beta blockers with a high extraction ratio. A reduced dose of beta-adrenergic blockers may be needed when a hyperthyroid patient becomes euthyroid.
Digitalis glycosides: Serum digitalis levels may be increased when hyperthyroid patients on a stable digitalis glycoside regimen become euthyroid; a reduced dose of digitalis glycosides may be needed.
Theophylline: Theophylline clearance may decrease when hyperthyroid patients on a stable theophylline regimen become euthyroid; a reduced dose of theophylline may be needed.
DESCRIPTION
Propylthiouracil, USP is one of the thiocarbamide compounds. It is a white, powdery, crystalline substance that has a bitter taste and is very slightly soluble in water. Propylthiouracil is an antithyroid drug administered orally. The structural formula is:
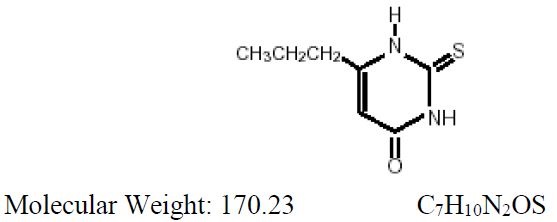
Each tablet contains propylthiouracil, USP 50 mg and the following inactive ingredients: colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, povidone, and pregelatinized corn starch,.
CLINICAL PHARMACOLOGY
Propylthiouracil inhibits the synthesis of thyroid hormones and thus is effective in the treatment of hyperthyroidism. The drug does not inactivate existing thyroxine and triiodothyronine that are stored in the thyroid or circulating in the blood, nor does it interfere with the effectiveness of thyroid hormones given by mouth or by injection. Propylthiouracil inhibits the conversion of thyroxine to triiodothyronine in peripheral tissues and may therefore be an effective treatment for thyroid storm.
Propylthiouracil is readily absorbed and is extensively metabolized. Approximately 35% of the drug is excreted in the urine, in intact and conjugated forms, within 24 hours.
HOW SUPPLIED
Propylthiouracil tablets, USP are available as follows:
50 mg — Each white, round, tablet imprinted with  on one side and 348 and partial bisect on the other side contains 50 mg of propylthiouracil, USP. Tablets are supplied in bottles of 100 (NDC 0228-2348-10).
on one side and 348 and partial bisect on the other side contains 50 mg of propylthiouracil, USP. Tablets are supplied in bottles of 100 (NDC 0228-2348-10).
Dispense in a well-closed container as defined in the USP.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].