Sodium Nitroprusside Prescribing Information
Continuously monitor blood pressure in patients receiving sodium nitroprusside. Start infusion of sodium nitroprusside at a rate of 0.3 mcg/kg/min. Evaluate blood pressure for at least 5 minutes before titrating to a higher or lower dose to achieve the desired blood pressure. The dose may be titrated upward until:
•the desired effect is achieved,
•systemic blood pressure cannot be further reduced without compromising the perfusion of vital organs, or
•the maximum recommended infusion rate of 10 mcg/kg/min has been reached, whichever occurs first.
In patients with eGFR <30 mL/min/1.73 m2, limit the mean infusion rate to less than 3 mcg/kg/min. In anuric patients, limit the mean infusion rate to 1 mcg/kg/min.
Sodium nitroprusside, can cause excessive hypotension leading to hypoperfusion of vital organs. Hypotension should resolve within 1-10 minutes after discontinuation of the nitroprusside infusion; during these few minutes, it may be helpful to put the patient into a head-down (Trendelenburg) position to maximize venous return. If hypotension persists more than a few minutes after discontinuation, consider other causes. Elderly patients may be more sensitive to the hypotensive effects of the drug.
Sodium nitroprusside infusions above 2 mcg/kg/min generate cyanide ion (CN¯) faster than the body can normally dispose of it. At the maximum recommended infusion rate of 10 mcg/kg/min, the patient’s ability to buffer CN¯ will be exceeded in less than one hour
Patients with hepatic dysfunction are more susceptible to cyanide toxicity.
An early manifestation of cyanide toxicity is increasing dosage requirements to maintain blood pressure control. Metabolic acidosis may not be evident for more than an hour after toxic cyanide levels accumulate.
If cyanide toxicity develops, discontinue sodium nitroprusside, and consider specific treatment of cyanide toxicity
Sodium nitroprusside is a direct acting vasodilator indicated for:
•Immediate reduction of blood pressure (
Sodium nitroprusside is indicated for the immediate reduction of blood pressure of adult and pediatric patients in hypertensive crises.
•Producing controlled hypotension to reduce bleeding during surgery. (
Sodium nitroprusside indicated for induction and maintenance of controlled hypotension in adults and children during surgery, to reduce bleeding.
•Treatment of acute heart failure to reduce left ventricular end-diastolic pressure, pulmonary capillary wedge pressure, peripheral vascular resistance and mean arterial blood pressure. (
Sodium nitroprusside is indicated for the treatment of acute heart failure to reduce, left ventricular end-diastolic pressure, pulmonary capillary wedge pressure, peripheral vascular resistance and mean arterial blood pressure.
Initiate infusion of sodium nitroprusside at a rate of 0.3 mcg/kg/min, and titrate every few minutes until the desired effect is achieved OR the maximum recommended infusion rate of 10 mcg/kg/min has been reached (
Continuously monitor blood pressure in patients receiving sodium nitroprusside. Start infusion of sodium nitroprusside at a rate of 0.3 mcg/kg/min. Evaluate blood pressure for at least 5 minutes before titrating to a higher or lower dose to achieve the desired blood pressure. The dose may be titrated upward until:
•the desired effect is achieved,
•systemic blood pressure cannot be further reduced without compromising the perfusion of vital organs, or
•the maximum recommended infusion rate of 10 mcg/kg/min has been reached, whichever occurs first.
In patients with eGFR <30 mL/min/1.73 m2, limit the mean infusion rate to less than 3 mcg/kg/min. In anuric patients, limit the mean infusion rate to 1 mcg/kg/min.
Injection: 50 mg/100 mL of 0.9% sodium chloride (0.5 mg/mL), 20 mg/100 mL of 0.9% sodium chloride (0.2 mg/mL), and 10 mg/50 mL of 0.9% sodium chloride (0.2 mg/mL). Sodium Nitroprusside in 0.9% Sodium Chloride Injection, ready to use is supplied as a sterile, unpreserved, colorless to red-brown solution available in a single-dose vial.
Based on animal data and mechanism of action, sodium nitroprusside may lead to cyanide exposure and potential adverse effects in the fetus
Infused sodium nitroprusside is rapidly distributed to a volume that is approximately coextensive with the extracellular space. The drug is cleared by intraerythrocytic reaction with hemoglobin (Hgb), and sodium nitroprusside’s resulting circulatory half-life is about 2 minutes.
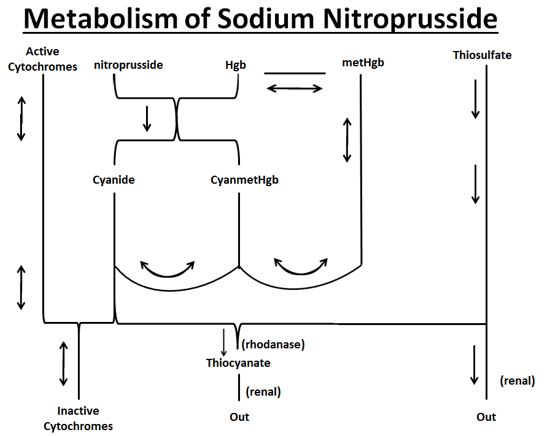
The products of the nitroprusside/hemoglobin reaction are cyanmethemoglobin (cyanmetHgb) and cyanide ion (CN¯).
As shown in the diagram below, the essential features of nitroprusside metabolism are:
•one molecule of sodium nitroprusside is metabolized by combination with hemoglobin to produce one molecule of cyanmethemoglobin and four CN¯ ions;
•methemoglobin, obtained from hemoglobin, can sequester cyanide as cyanmethemoglobin;
•thiosulfate reacts with cyanide to produce thiocyanate;
•thiocyanate is eliminated in the urine;
•cyanide not otherwise removed binds to cytochromes; and
•cyanide is much more toxic than methemoglobin or thiocyanate.
Cyanide ion is normally found in serum; it is derived from dietary substrates and from tobacco smoke. CN¯ levels in packed erythrocytes are typically less than 1 μmol/L (less than 25 mcg/L); levels are roughly doubled in heavy smokers.
At healthy steady state, most people have less than 1% of their hemoglobin in the form of methemoglobin. Nitroprusside metabolism can lead to methemoglobin formation. Relatively large quantities of sodium nitroprusside, however, are required to produce significant methemoglobinemia.
At physiologic methemoglobin levels, the CN¯ binding capacity of packed red cells is a little less than 200 μmol/L (5 mg/L). Cytochrome toxicity is seen at levels only slightly higher, and death has been reported at levels from 300 to 3000 μmol/L (8–80 mg/L). A patient with a normal redcell mass (35 mL/kg) and normal methemoglobin levels can buffer about 175 mcg/kg of CN¯, corresponding to a little less than 500 mcg/kg of infused sodium nitroprusside.
Thiocyanate (SCN¯) is a normal physiological constituent of serum, with normal levels typically in the range of 50-250 μmol/L (3-15 mg/L). Clearance of SCN¯ is primarily renal. In renal failure, the half-life can be doubled or tripled.
When thiosulfate is being supplied only by normal physiologic mechanisms, conversion of CN¯ to SCN¯ generally proceeds at about 1 mcg/kg/min. This rate of CN¯ clearance corresponds to steady-state processing of a sodium nitroprusside infusion of slightly more than 2 mcg/kg/min. CN¯ begins to accumulate when sodium nitroprusside infusions exceed this rate.

The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Prolonged use and large doses of sodium nitroprusside during pregnancy may result in cyanide toxicity that may be fatal to the fetus. In the unusual case that there is no appropriate alternative to therapy with sodium nitroprusside for a particular patient, apprise the mother of the potential risk to the fetus
Sodium nitroprusside infusions above 2 mcg/kg/min generate cyanide ion (CN¯) faster than the body can normally dispose of it. At the maximum recommended infusion rate of 10 mcg/kg/min, the patient’s ability to buffer CN¯ will be exceeded in less than one hour
Patients with hepatic dysfunction are more susceptible to cyanide toxicity.
An early manifestation of cyanide toxicity is increasing dosage requirements to maintain blood pressure control. Metabolic acidosis may not be evident for more than an hour after toxic cyanide levels accumulate.
If cyanide toxicity develops, discontinue sodium nitroprusside, and consider specific treatment of cyanide toxicity
A small number of cases have reported adverse events, including stillbirths, in pregnant women with severe pregnancy-induced hypertension who were treated with sodium nitroprusside. However, methodological limitations, including small sample size and limited information on sodium nitroprusside dosage and duration of treatment, as well as the cyanide concentration in maternal blood or fetal tissue, preclude a reliable evaluation of the potential risk of adverse fetal outcomes with the use of sodium nitroprusside during pregnancy.
In three studies in pregnant ewes, nitroprusside was shown to cross the placental barrier. Fetal cyanide levels were shown to be dose-related to maternal levels of nitroprusside. The metabolic transformation of sodium nitroprusside given to pregnant ewes led to fatal levels of cyanide in the fetuses. The infusion of 25 mcg/kg/min of sodium nitroprusside for one hour in pregnant ewes resulted in the death of all fetuses. Pregnant ewes infused with 1 mcg/kg/min of sodium nitroprusside for one hour delivered normal lambs.
- Diseases with compensatory hypertension (e.g., coarctation of the aorta, arteriovenous shunting).
- Inadequate cerebral circulation or in moribund patients (A.S.A. Class 5E) coming to emergency surgery.
- Patients with congenital (Leber’s) optic atrophy or with tobacco amblyopia.
- Acute heart failure associated with reduced peripheral vascular resistance.
- Concomitant use with sildenafil, tadalafil, vardenifil, or riociguat.