Get your patient on Stiolto Respimat (Tiotropium Bromide And Olodaterol)
Stiolto Respimat prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Stiolto Respimat patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- For oral inhalation only.
- Two inhalations of STIOLTO RESPIMAT once-daily at the same time of day. (2 )
Recommended Dosage
The recommended dosage of STIOLTO RESPIMAT is two inhalations once-daily at the same time of the day. Do not use STIOLTO RESPIMAT more than two inhalations every 24 hours.
Administration Information
For oral inhalation only.
Prior to first use, the STIOLTO RESPIMAT cartridge is inserted into the STIOLTO RESPIMAT inhaler and the unit is primed. When using the unit for the first time, patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use [see Patient Counseling Information (17) ] .
No dosage adjustment is required for geriatric, hepatically-impaired, or renally-impaired patients. However, patients with moderate to severe renal impairment given STIOLTO RESPIMAT should be monitored closely for anticholinergic effects [see Warnings and Precautions (5.10) , Use in Specific Populations (8.5 , 8.6 , 8.7) , and Clinical Pharmacology (12.3) ] .

By using PrescriberAI, you agree to the AI Terms of Use.
Stiolto Respimat prescribing information
INDICATIONS AND USAGE
STIOLTO RESPIMAT is a combination of tiotropium bromide, an anticholinergic and olodaterol, a long-acting beta 2 -adrenergic agonist (LABA) indicated for the long-term, once-daily maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). (1.1 )
Important limitations:
Maintenance Treatment of COPD
STIOLTO RESPIMAT is a combination of tiotropium bromide and olodaterol indicated for long-term, once-daily maintenance treatment of patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Important Limitations of Use
- STIOLTO RESPIMAT is not indicated to treat acute deteriorations of COPD [see Warnings and Precautions (5.2) ].
- STIOLTO RESPIMAT is not indicated to treat asthma. The safety and effectiveness of STIOLTO RESPIMAT in asthma have not been established.
DOSAGE AND ADMINISTRATION
- For oral inhalation only.
- Two inhalations of STIOLTO RESPIMAT once-daily at the same time of day. (2 )
Recommended Dosage
The recommended dosage of STIOLTO RESPIMAT is two inhalations once-daily at the same time of the day. Do not use STIOLTO RESPIMAT more than two inhalations every 24 hours.
Administration Information
For oral inhalation only.
Prior to first use, the STIOLTO RESPIMAT cartridge is inserted into the STIOLTO RESPIMAT inhaler and the unit is primed. When using the unit for the first time, patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use [see Patient Counseling Information (17) ] .
No dosage adjustment is required for geriatric, hepatically-impaired, or renally-impaired patients. However, patients with moderate to severe renal impairment given STIOLTO RESPIMAT should be monitored closely for anticholinergic effects [see Warnings and Precautions (5.10) , Use in Specific Populations (8.5 , 8.6 , 8.7) , and Clinical Pharmacology (12.3) ] .
DOSAGE FORMS AND STRENGTHS
Inhalation spray: Each actuation from the mouthpiece delivers 2.5 mcg tiotropium (equivalent to 3.124 mcg tiotropium bromide monohydrate), and 2.5 mcg olodaterol (equivalent to 2.736 mcg olodaterol hydrochloride). Two actuations equal one dose. (3 )
Inhalation Spray: STIOLTO RESPIMAT consists of a STIOLTO RESPIMAT inhaler and an aluminum cylinder (STIOLTO RESPIMAT cartridge) containing a combination of tiotropium bromide (as the monohydrate) and olodaterol (as the hydrochloride). The STIOLTO RESPIMAT cartridge is intended only for use with the STIOLTO RESPIMAT inhaler.
Each actuation from the STIOLTO RESPIMAT inhaler delivers 2.5 mcg tiotropium (equivalent to 3.124 mcg tiotropium bromide monohydrate) and 2.5 mcg olodaterol (equivalent to 2.736 mcg olodaterol hydrochloride) from the mouthpiece.
Two actuations equal one dose.
USE IN SPECIFIC POPULATIONS
Patients with moderate to severe renal impairment should be monitored closely for potential anticholinergic side effects. (2 , 8.7 )
Pregnancy
Risk Summary
There are no adequate and well-controlled clinical studies with STIOLTO RESPIMAT or its individual components, tiotropium bromide and olodaterol, in pregnant women to inform of drug-associated risk of adverse pregnancy-related outcomes. Animal reproduction studies were conducted with the individual components of STIOLTO RESPIMAT, tiotropium bromide and olodaterol. There are clinical considerations with the use of STIOLTO RESPIMAT in pregnant women (see Clinical Considerations ) . STIOLTO RESPIMAT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Based on animal reproduction studies, no structural abnormalities were observed when tiotropium was administered by inhalation to pregnant rats and rabbits during the period of organogenesis at doses 790 and 8 times, respectively, the maximum recommended human daily inhalation dose (MRHDID). Increased post-implantation loss was observed in rats and rabbits administered tiotropium at maternally toxic doses 430 times and 40 times the MRHDID, respectively (see Data ) . Based on animal studies, olodaterol was not teratogenic when administered to pregnant rats or rabbits during organogenesis at inhalation doses of approximately 2,731 or 1,353 times the MRHDID (on an AUC basis), in rats or rabbits, respectively (see Data ) .
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Labor and Delivery
There are no adequate and well-controlled human studies that have investigated the effects of STIOLTO RESPIMAT on preterm labor or labor at term. Because of the potential for beta-agonist interference with uterine contractility, use of STIOLTO RESPIMAT during labor should be restricted to those patients in whom the benefits clearly outweigh the risks.
Data
Animal Data
Animal reproduction studies with the combination of tiotropium and olodaterol are not available; however, studies are available with the individual components.
Tiotropium
In 2 separate embryo-fetal development studies, pregnant rats and rabbits received tiotropium during the period of organogenesis at doses up to approximately 790 and 8 times the MRHDID, respectively (on a mcg/m 2 basis at inhalation doses of 1,471 and 7 mcg/kg/day in rats and rabbits, respectively). No evidence of structural abnormalities was observed in rats or rabbits. However, in rats, tiotropium caused fetal resorption, litter loss, decreases in the number of live pups at birth and the mean pup weights, and a delay in pup sexual maturation at tiotropium doses of approximately 40 times the MRHDID (on a mcg/m 2 basis at a maternal inhalation dose of 78 mcg/kg/day). In rabbits, tiotropium caused an increase in post-implantation loss at a tiotropium dose of approximately 430 times the MRHDID (on a mcg/m 2 basis at a maternal inhalation dose of 400 mcg/kg/day). Such effects were not observed at approximately 5 and 95 times the MRHDID, respectively (on a mcg/m 2 basis at inhalation doses of 9 and 88 mcg/kg/day in rats and rabbits, respectively).
Olodaterol
Olodaterol was not teratogenic in rats at inhalation doses approximately 2,731 times the MRHDID (on an AUC basis at a maternal inhalation dose of 1,054 mcg/kg/day). No significant effects occurred in rabbits at inhalation doses approximately 1,353 times the MRHDID in adults (on an AUC basis at a maternal inhalation dose of 974 mcg/kg/day). Placental transfer of olodaterol was observed in pregnant rats.
Olodaterol has been shown to be teratogenic in New Zealand rabbits at inhalation doses approximately 7,130 times the MRHDID in adults (on an AUC basis at a maternal inhalation dose of 2,489 mcg/kg/day). Olodaterol exhibited the following fetal toxicities: enlarged or small heart atria or ventricles, eye abnormalities, and split or distorted sternum.
Lactation
Risk Summary
There are no data on the presence of tiotropium or olodaterol in human milk, the effects on the breastfed infant, or the effects on milk production. Tiotropium, olodaterol, and/or their metabolites are present in the milk of lactating rats, however, due to species-specific differences in lactation physiology, the clinical relevance of these data are not clear (see Data ) . The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for STIOLTO RESPIMAT and any potential adverse effects on the breastfed child from STIOLTO RESPIMAT or from the underlying maternal condition.
Data
The distributions of tiotropium bromide or olodaterol into milk were investigated in separate studies after a single intravenous administration of 10 mg/kg or 0.4 μmol/kg, respectively, to lactating rats. Tiotropium, olodaterol, and/or their metabolites are present in the milk of lactating rats at concentrations above those in plasma.
Pediatric Use
COPD does not normally occur in children. The safety and effectiveness of STIOLTO RESPIMAT in the pediatric population has not been established.
Geriatric Use
Based on available data, no adjustment of STIOLTO RESPIMAT dosage in geriatric patients is warranted [see Clinical Pharmacology (12.3) ].
Of the 1,029 patients who received STIOLTO RESPIMAT at the recommended dose once daily in the clinical studies from the pooled 1-year database, 525 (51.0%) were <65 years of age, 407 (39.6%) were 65 to <75, 96 (9.3%) were 75 to <85, and 1 (0.1%) was ≥85.
No overall differences in effectiveness were observed, and in the 1-year pooled data, the adverse drug reaction profiles were similar in the older population compared to the patient population overall.
Hepatic Impairment
No dose adjustment is needed in patients with mild and moderate hepatic impairment. A study in subjects with severe hepatic impairment was not performed [see Clinical Pharmacology (12.3) ] .
Renal Impairment
No dose adjustment is required for patients with renal impairment. However, patients with moderate to severe renal impairment (creatinine clearance of <60 mL/min) treated with STIOLTO RESPIMAT should be monitored closely for anticholinergic side effects [see Dosage and Administration (2) , Warnings and Precautions (5.10) , and Clinical Pharmacology (12.3) ] .
CONTRAINDICATIONS
Use of a LABA, including STIOLTO RESPIMAT, without an inhaled corticosteroid is contraindicated in patients with asthma [see Warnings and Precautions (5.1) ] . STIOLTO RESPIMAT is not indicated for the treatment of asthma.
STIOLTO RESPIMAT is contraindicated in patients with a hypersensitivity to tiotropium, ipratropium, olodaterol, or any component of this product [see Warnings and Precautions (5.4) ] .
In clinical trials and postmarketing experience with tiotropium, immediate hypersensitivity reactions, including angioedema (including swelling of the lips, tongue, or throat), itching, or rash have been reported. Hypersensitivity reactions were also reported in clinical trials with STIOLTO RESPIMAT.
WARNINGS AND PRECAUTIONS
- LABA as monotherapy (without an inhaled corticosteroid) for asthma increases the risk of serious asthma-related events. (5.1 )
- Do not initiate STIOLTO RESPIMAT in acutely deteriorating COPD patients. (5.2 )
- Do not use for relief of acute symptoms. Concomitant short-acting beta 2 -agonists can be used as needed for acute relief. (5.2 )
- Do not exceed the recommended dosage. Excessive use of STIOLTO RESPIMAT, or use in conjunction with other medications containing LABA can result in clinically significant cardiovascular effects and may be fatal. (5.3 )
- Immediate hypersensitivity reactions: Discontinue STIOLTO RESPIMAT at once and consider alternatives if immediate hypersensitivity reactions, including angioedema, urticaria, rash, bronchospasm, or anaphylaxis, occur. (5.4 )
- Life-threatening paradoxical bronchospasm can occur. Discontinue STIOLTO RESPIMAT immediately. (5.5 )
- Use with caution in patients with cardiovascular or convulsive disorders, thyrotoxicosis, or sensitivity to sympathomimetic drugs. (5.6 , 5.7 )
- Worsening of narrow-angle glaucoma may occur. Use with caution in patients with narrow-angle glaucoma and instruct patients to consult a physician immediately if this occurs. (5.8 )
- Worsening of urinary retention may occur. Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction and instruct patients to consult a physician immediately if this occurs. (5.9 )
- Be alert to hypokalemia and hyperglycemia. (5.11 )
Serious Asthma-Related Events – Hospitalizations, Intubations, Death
- The safety and efficacy of STIOLTO RESPIMAT in patients with asthma have not been established. STIOLTO RESPIMAT is not indicated for the treatment of asthma [see Contraindications (4) ] .
- Use of long-acting beta 2 -adrenergic agonists (LABA) as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
- A 28-week, placebo-controlled US study comparing the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in patients receiving salmeterol (13/13,176 in patients treated with salmeterol vs. 3/13,179 in patients treated with placebo; RR 4.37, 95% CI 1.25, 15.34). The increased risk of asthma-related death is considered a class effect of LABA, including olodaterol, one of the active ingredients in STIOLTO RESPIMAT.
- No study adequate to determine whether the rate of asthma-related death is increased in patients treated with STIOLTO RESPIMAT has been conducted.
- Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
Deterioration of Disease and Acute Episodes
STIOLTO RESPIMAT should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition. STIOLTO RESPIMAT has not been studied in patients with acutely deteriorating COPD. The use of STIOLTO RESPIMAT in this setting is inappropriate.
STIOLTO RESPIMAT should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. STIOLTO RESPIMAT has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled short-acting beta 2 -agonist.
When beginning STIOLTO RESPIMAT, patients who have been taking inhaled, short-acting beta 2 -agonists on a regular basis (e.g., four times a day) should be instructed to discontinue the regular use of these drugs and use them only for symptomatic relief of acute respiratory symptoms. When prescribing STIOLTO RESPIMAT, the healthcare provider should also prescribe an inhaled, short-acting beta 2 -agonist and instruct the patient on how it should be used. Increasing inhaled beta 2 -agonist use is a signal of deteriorating disease for which prompt medical attention is indicated.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If STIOLTO RESPIMAT no longer controls symptoms of bronchoconstriction, or the patient's inhaled, short-acting beta 2 -agonist becomes less effective or the patient needs more inhalation of short-acting beta 2 -agonist than usual, these may be markers of deterioration of disease. In this setting, a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dosage of STIOLTO RESPIMAT beyond the recommended dosage is not appropriate in this situation.
Excessive Use of STIOLTO RESPIMAT and Use With Other Long-Acting Beta 2 -Agonists
As with other inhaled drugs containing beta 2 -adrenergic agents, STIOLTO RESPIMAT should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing long-acting beta 2 -agonists, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions, including urticaria, angioedema (including swelling of the lips, tongue or throat), rash, bronchospasm, anaphylaxis, or itching may occur after administration of STIOLTO RESPIMAT. If such a reaction occurs, therapy with STIOLTO RESPIMAT should be stopped at once and alternative treatments should be considered. Given the similar structural formula of atropine to tiotropium, patients with a history of hypersensitivity reactions to atropine or its derivatives should be closely monitored for similar hypersensitivity reactions to STIOLTO RESPIMAT.
Paradoxical Bronchospasm
As with other inhaled medicines, STIOLTO RESPIMAT may cause paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs, STIOLTO RESPIMAT should be stopped immediately and alternative therapy instituted.
Cardiovascular Effects
Olodaterol, like other beta 2 -agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and/or symptoms. If such effects occur, STIOLTO RESPIMAT may need to be discontinued. In addition, beta-agonists have been reported to produce ECG changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Long acting beta 2 -adrenergic agonists should be administered with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, hypertrophic obstructive cardiomyopathy, and hypertension.
Coexisting Conditions
Olodaterol, like other sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis, in patients with known or suspected prolongation of the QT interval, and in patients who are unusually responsive to sympathomimetic amines. Doses of the related beta 2 -agonist albuterol, when administered intravenously, have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis.
Worsening of Narrow-Angle Glaucoma
STIOLTO RESPIMAT should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
Worsening of Urinary Retention
STIOLTO RESPIMAT should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of prostatic hyperplasia or bladder-neck obstruction (e.g., difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder neck obstruction. Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
Renal Impairment
Because tiotropium is a predominantly renally excreted drug, patients with moderate to severe renal impairment (creatinine clearance of <60 mL/min) treated with STIOLTO RESPIMAT should be monitored closely for anticholinergic side effects [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3) ] .
Hypokalemia and Hyperglycemia
Beta-adrenergic agonists may produce significant hypokalemia in some patients, which has the potential to produce adverse cardiovascular effects [see Clinical Pharmacology (12.2) ] . The decrease in serum potassium is usually transient, not requiring supplementation. Inhalation of high doses of beta 2 -adrenergic agonists may produce increases in plasma glucose.
In patients with severe COPD, hypokalemia may be potentiated by hypoxia and concomitant treatment [see Drug Interactions (7.2) ] , which may increase the susceptibility for cardiac arrhythmias.
Clinically notable decreases in serum potassium or changes in blood glucose were infrequent during clinical studies with long-term administration of olodaterol with the rates similar to those for placebo controls. Olodaterol has not been investigated in patients whose diabetes mellitus is not well controlled.
ADVERSE REACTIONS
LABA, such as olodaterol, one of the active components in STIOLTO RESPIMAT, as monotherapy (without an inhaled corticosteroid) for asthma, increase the risk of asthma-related events. STIOLTO RESPIMAT is not indicated for the treatment of asthma [see Warning and Precautions (5.1) ].
The following adverse reactions are described, or described in greater detail, in other sections:
- Immediate hypersensitivity reactions [see Warnings and Precautions (5.4) ]
- Paradoxical bronchospasm [see Warnings and Precautions (5.5) ]
- Worsening of narrow-angle glaucoma [see Warnings and Precautions (5.8) ]
- Worsening of urinary retention [see Warnings and Precautions (5.9) ]
Clinical Trials Experience in Chronic Obstructive Pulmonary Disease
Because clinical trials are conducted under widely varying conditions, the incidence of adverse reactions observed in the clinical trials of a drug cannot be directly compared to the incidences in the clinical trials of another drug and may not reflect the incidences observed in practice.
The clinical program for STIOLTO RESPIMAT included 7,151 subjects with COPD in two 52-week active-controlled trials, one 12-week placebo-controlled trial, three 6-week placebo-controlled cross-over trials, and four additional trials of shorter duration. A total of 1,988 subjects received at least 1 dose of STIOLTO RESPIMAT. Adverse reactions observed in the ≤12-week trials were consistent with those observed in the 52-week trials, which formed the primary safety database.
The primary safety database consisted of pooled data from the two 52-week double-blind, active-controlled, parallel group confirmatory clinical trials (Trials 1 and 2). These trials included 5,162 adult COPD patients (72.9% males and 27.1% females) 40 years of age and older. Of these patients, 1,029 were treated with STIOLTO RESPIMAT once daily. The STIOLTO RESPIMAT group was composed of mostly Caucasians (71.1%) with a mean age of 63.8 years and a mean percent predicted FEV 1 at baseline of 43.2%. In these two trials, tiotropium 5 mcg and olodaterol 5 mcg were included as active control arms and no placebo was used.
In these two clinical trials, 74% of patients exposed to STIOLTO RESPIMAT reported an adverse reaction compared to 76.6% and 73.3% in the olodaterol 5 mcg and tiotropium 5 mcg groups, respectively. The proportion of patients who discontinued due to an adverse reaction was 7.4% for STIOLTO RESPIMAT treated patients compared to 9.9% and 9.0% for olodaterol 5 mcg and tiotropium 5 mcg treated patients. The adverse reaction most commonly leading to discontinuation was worsening COPD.
The most common serious adverse reactions were COPD exacerbation and pneumonia.
Table 1 shows all adverse drug reactions that occurred with an incidence of >3% in the STIOLTO RESPIMAT treatment group and a higher incidence rate than the active comparator groups listed.
| Treatment | STIOLTO RESPIMAT (once daily) | Tiotropium (5 mcg once daily) | Olodaterol (5 mcg once daily) |
|---|---|---|---|
| Body system (adverse drug reaction) | n=1,029 n (%) | n=1,033 n (%) | n=1,038 n (%) |
| Infections and infestations | |||
| Nasopharyngitis | 128 (12.4) | 121 (11.7) | 131 (12.6) |
| Respiratory, thoracic, and mediastinal disorders | |||
| Cough | 40 (3.9) | 45 (4.4) | 31 (3.0) |
| Musculoskeletal and connective tissue disorders | |||
| Back Pain | 37 (3.6) | 19 (1.8) | 35 (3.4) |
Other adverse drug reactions in patients receiving STIOLTO RESPIMAT that occurred in ≤3% of patients in clinical studies are listed below:
Metabolism and nutrition disorders: dehydration Nervous system disorders: dizziness, insomnia Eye disorders: glaucoma, intraocular pressure increased, vision blurred Cardiac/vascular disorders: atrial fibrillation, palpitations, supraventricular tachycardia, tachycardia, hypertension Respiratory, thoracic, and mediastinal disorders: epistaxis, pharyngitis, dysphonia, bronchospasm, laryngitis, sinusitis Gastrointestinal disorders: dry mouth, constipation, oropharyngeal candidiasis, dysphagia, gastroesophageal reflux disease, gingivitis, glossitis, stomatitis, intestinal obstruction including ileus paralytic Skin and subcutaneous disorders: rash, pruritus, angioneurotic edema, urticaria, skin infection, and skin ulcer, dry skin, hypersensitivity (including immediate reactions) Musculoskeletal and connective tissue disorders: arthralgia, joint swelling Renal and urinary disorders: urinary retention, dysuria, and urinary tract infection
COPD Exacerbation Reduction Trial
In a one year trial (Trial 5) of 7,880 patients to compare rates of COPD exacerbations, 3,939 patients were treated with STIOLTO RESPIMAT and 3,941 patients were treated with tiotropium 5 mcg inhalation spray. The safety profile of STIOLTO RESPIMAT was similar to that of tiotropium 5 mcg inhalation spray and consistent with that documented in the STIOLTO RESPIMAT primary safety database.
DRUG INTERACTIONS
- Other adrenergic drugs may potentiate effect. Use with caution. (5.3 , 7.1 )
- Xanthine derivatives, steroids, diuretics, or non-potassium sparing diuretics may potentiate hypokalemia or ECG changes. Use with caution. (7.2 , 7.3 )
- MAO inhibitors, tricyclic antidepressants, and drugs that prolong QTc interval may potentiate effect on cardiovascular system. Use with extreme caution. (7.4 )
- Beta-blockers may decrease effectiveness. Use with caution and only when medically necessary. (7.5 )
- Anticholinergics: May interact additively with concomitantly used anticholinergic medications. Avoid administration of STIOLTO RESPIMAT with other anticholinergic-containing drugs. (7.6 )
Adrenergic Drugs
If additional adrenergic drugs are to be administered by any route, they should be used with caution because the sympathetic effects of olodaterol, one component of STIOLTO RESPIMAT, may be potentiated [see Warnings and Precautions (5.3 , 5.6 , 5.10 , 5.11) ] .
Sympathomimetics, Xanthine Derivatives, Steroids, or Diuretics
Tiotropium has been used concomitantly with short-acting and long-acting sympathomimetic (beta-agonists) bronchodilators, methylxanthines, and oral and inhaled steroids, without increases in adverse reactions. Concomitant treatment with xanthine derivatives, steroids, or diuretics may potentiate any hypokalemic effect of olodaterol [see Warnings and Precautions (5.11) ] .
Non-Potassium Sparing Diuretics
The ECG changes and/or hypokalemia that may result from the administration of non-potassium sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dosage of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the co-administration of STIOLTO RESPIMAT with non-potassium sparing diuretics.
Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, QTc Prolonging Drugs
STIOLTO RESPIMAT, as with other drugs containing beta 2 -agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants or other drugs known to prolong the QTc interval because the action of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval may be associated with an increased risk of ventricular arrhythmias.
Beta-Blockers
Beta-adrenergic receptor antagonists (beta-blockers) and the olodaterol component of STIOLTO RESPIMAT may interfere with the effect of each other when administered concurrently. Beta-blockers not only block the therapeutic effects of beta-agonists, but may produce severe bronchospasm in COPD patients. Therefore, patients with COPD should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-blockers in patients with COPD. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
Anticholinergics
There is potential for an additive interaction with concomitantly used anticholinergic medications. Therefore, avoid co-administration of STIOLTO RESPIMAT with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects [see Warnings and Precautions (5.8 , 5.9) and Adverse Reactions (6) ] .
Inhibitors of Cytochrome P450 and P-gp Efflux Transporter
In a drug interaction study using the strong dual CYP and P-gp inhibitor ketoconazole, a 1.7-fold increase of olodaterol maximum plasma concentrations and AUC was observed [see Pharmacokinetics (12.3) ] . Olodaterol was evaluated in clinical trials for up to one year at doses up to twice the recommended therapeutic dosage. No dosage adjustment of STIOLTO RESPIMAT is necessary.
DESCRIPTION
STIOLTO RESPIMAT is a combination of tiotropium, an anticholinergic, and olodaterol, a long-acting beta 2 -adrenergic agonist (LABA).
The drug substance tiotropium bromide monohydrate is chemically described as (1α, 2ß, 4ß, 5α, 7ß)-7-[(Hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.0 2,4 ] nonane bromide monohydrate. It is a synthetic, non-chiral, quaternary ammonium compound. Tiotropium bromide is a white or yellowish white powder. It is sparingly soluble in water and soluble in methanol.
The structural formula is:
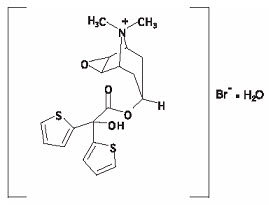
Tiotropium bromide (monohydrate) has a molecular mass of 490.4 and a molecular formula of C 19 H 22 NO 4 S 2 Br ∙ H 2 O.
The drug substance olodaterol hydrochloride is chemically described as 2H-1,4-Benzoxazin-3H(4H)-one, 6-hydroxy-8-[(1R)-1-hydroxy-2-[[2-(4-methoxyphenyl)-1,1-dimethylethyl]-amino]ethyl]-, monohydrochloride. Olodaterol hydrochloride is a white to off-white powder that is sparingly-slightly soluble in water and slightly soluble in ethanol. The molecular weight is 422.9 g/mole (salt): 386.5 g/mole (base), and the molecular formula is C 21 H 26 N 2 O 5 × HCl as a hydrochloride. The conversion factor from salt to free base is 1.094.
The structural formula is:
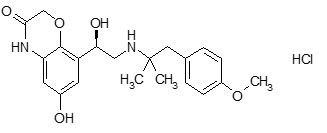
The drug product, STIOLTO RESPIMAT, is composed of a sterile aqueous solution of tiotropium bromide and olodaterol hydrochloride filled into a 4.5 mL plastic container crimped into an aluminum cylinder (STIOLTO RESPIMAT cartridge) for use with the STIOLTO RESPIMAT inhaler.
Excipients include, benzalkonium chloride, edetate disodium, hydrochloric acid, and water for injection.
The STIOLTO RESPIMAT cartridge is only intended for use with the STIOLTO RESPIMAT inhaler. The STIOLTO RESPIMAT inhaler is a hand held, pocket sized oral inhalation device that uses mechanical energy to generate a slow-moving aerosol cloud of medication from a metered volume of the drug solution. The STIOLTO RESPIMAT inhaler has a light green-colored cap.
When used with the STIOLTO RESPIMAT inhaler each cartridge, containing 4 grams of sterile aqueous solution, delivers the labeled number of metered actuations after preparation for use. Each dose (one dose equals two actuations) from the STIOLTO RESPIMAT inhaler delivers 5 mcg tiotropium (equivalent to 6.247 mcg tiotropium bromide monohydrate) and 5 mcg olodaterol (equivalent to 5.473 mcg olodaterol hydrochloride) in 22.1 mcL from the mouthpiece. As with all inhaled drugs, the actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between the actuation of the inhaler and inspiration through the delivery system. The duration of inspiration should be at least as long as the spray duration (1.5 seconds).
CLINICAL PHARMACOLOGY
Mechanism of Action
STIOLTO RESPIMAT
STIOLTO RESPIMAT contains both tiotropium and olodaterol. The properties described below for the individual components apply to STIOLTO RESPIMAT. These drugs represent 2 different classes of medication (an anticholinergic and a beta-agonist) that have different effects on clinical and physiological indices.
Tiotropium
Tiotropium is a long-acting, muscarinic antagonist which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors, M 1 to M 5 . In the airways, it exhibits pharmacological effects through inhibition of M 3 -receptors at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methacholine-induced bronchoconstriction effects was dose-dependent and lasted longer than 24 hours. The bronchodilation following inhalation of tiotropium is predominantly a site-specific effect.
Olodaterol
Olodaterol is a long-acting beta 2 -adrenergic agonist (LABA). The compound exerts its pharmacological effects by binding and activation of beta 2 -adrenoceptors after topical administration by inhalation. Activation of these receptors in the airways results in a stimulation of intracellular adenyl cyclase, an enzyme that mediates the synthesis of cyclic-3', 5' adenosine monophosphate (cAMP). Elevated levels of cAMP induce bronchodilation by relaxation of airway smooth muscle cells. In vitro studies have shown that olodaterol has 241-fold greater agonist activity at beta 2 -adrenoceptors compared to beta 1 -adrenoceptors and 2,299-fold greater agonist activity compared to beta 3 -adrenoceptors. The clinical significance of these findings is unknown.
Beta-adrenoceptors are divided into three subtypes: beta 1 -adrenoceptors predominantly expressed on cardiac muscle, beta 2 -adrenoceptors predominantly expressed on airway smooth muscle, and beta 3 -adrenoceptors predominantly expressed on adipose tissue. Beta 2 -agonists cause bronchodilation. Although the beta 2 -adrenoceptor is the predominant adrenergic receptor in the airway smooth muscle, it is also present on the surface of a variety of other cells, including lung epithelial and endothelial cells and in the heart. The precise function of beta 2 -receptors in the heart is not known, but their presence raises the possibility that even highly selective beta 2 -agonists may have cardiac effects.
Pharmacodynamics
Cardiac Electrophysiology
STIOLTO RESPIMAT
In two 52-week randomized, double-blind trials using STIOLTO RESPIMAT that enrolled 5,162 patients with COPD, ECG assessments were performed post-dose on days 1, 85, 169, and 365. In a pooled analysis the number of subjects with changes from baseline-corrected QT interval of >30 msec using both the Bazett (QTcB) and Fredericia (QTcF), corrections of QT for heart rate were not different for the STIOLTO RESPIMAT group compared to olodaterol 5 mcg and tiotropium 5 mcg across the assessments conducted.
Tiotropium
The effect of tiotropium dry powder for inhalation on QT interval was also evaluated in a randomized, placebo- and positive-controlled crossover study in 53 healthy volunteers. Subjects received tiotropium inhalation powder 18 mcg, 54 mcg (3 times the recommended dose), or placebo for 12 days. ECG assessments were performed at baseline and throughout the dosing interval following the first and last dose of study medication. Relative to placebo, the maximum mean change from baseline in study-specific QTc interval was 3.2 msec and 0.8 msec for tiotropium inhalation powder 18 mcg and 54 mcg, respectively. No subject showed a new onset of QTc >500 msec or QTc changes from baseline of ≥60 msec.
In a multicenter, randomized, double-blind trial using tiotropium dry powder for inhalation that enrolled 198 patients with COPD, the number of subjects with changes from baseline-corrected QT interval of 30–60 msec was higher in the tiotropium group as compared with placebo. This difference was apparent using both the Bazett (QTcB) [20 (20%) patients vs. 12 (12%) patients] and Fredericia (QTcF) [16 (16%) patients vs. 1 (1%) patient] corrections of QT for heart rate. No patients in either group had either QTcB or QTcF of >500 msec. Other clinical trials with tiotropium did not detect an effect of the drug on QTc intervals.
Olodaterol
The effect of olodaterol on the QT/QTc interval of the ECG was investigated in 24 healthy male and female volunteers in a double-blind, randomized, placebo- and active (moxifloxacin)- controlled study at single doses of 10, 20, 30, and 50 mcg. Dose-dependent QtcI (individual subject corrected QT interval) prolongation was observed. The maximum mean (one-sided 95% upper confidence bound) difference in QTcI from placebo after baseline correction was 2.5 (5.6) ms, 6.1 (9.2) ms, 7.5 (10.7) ms, and 8.5 (11.6) ms following doses of 10, 20, 30, and 50 mcg, respectively.
The effect of 5 mcg and 10 mcg olodaterol on heart rate and rhythm was assessed using continuous 24-hour ECG recording (Holter monitoring) in a subset of 772 patients in the 48-week, placebo-controlled phase 3 trials. There were no dose- or time-related trends or patterns observed for the magnitudes of mean changes in heart rate or premature beats. Shifts from baseline to the end of treatment in premature beats did not indicate meaningful differences between olodaterol 5 mcg, 10 mcg, and placebo.
Pharmacokinetics
STIOLTO RESPIMAT
When STIOLTO RESPIMAT was administered by the inhalation route, the pharmacokinetic parameters for tiotropium and for olodaterol were similar to those observed when each active substance was administered separately.
Tiotropium
Tiotropium is administered as an inhalation spray. Some of the pharmacokinetic data described below were obtained with higher doses than recommended for therapy.
Olodaterol
Olodaterol showed linear pharmacokinetics. On repeated once-daily inhalation, steady-state of olodaterol plasma concentrations was achieved after 8 days, and the extent of exposure was increased up to 1.8-fold as compared to a single dose.
Absorption
Tiotropium
Following inhalation of the solution by young healthy volunteers, urinary excretion data suggests that approximately 33% of the inhaled dose reaches the systemic circulation. Oral solutions of tiotropium have an absolute bioavailability of 2% to 3%. Food is not expected to influence the absorption of tiotropium for the same reason. Maximum tiotropium plasma concentrations were observed 5 to 7 minutes after inhalation.
Olodaterol
Olodaterol reaches maximum plasma concentrations generally within 10 to 20 minutes following drug inhalation. In healthy volunteers the absolute bioavailability of olodaterol following inhalation was estimated to be approximately 30%, whereas the absolute bioavailability was below 1% when given as an oral solution. Thus, the systemic availability of olodaterol after inhalation is mainly determined by lung absorption, while any swallowed portion of the dose only negligibly contributes to systemic exposure.
Distribution
Tiotropium
The drug has a plasma protein binding of 72% and shows a volume of distribution of 32 L/kg. Local concentrations in the lung are not known, but the mode of administration suggests substantially higher concentrations in the lung. Studies in rats have shown that tiotropium does not penetrate the blood-brain barrier.
Olodaterol
Olodaterol exhibits multi-compartmental disposition kinetics after inhalation as well as after intravenous administration. The volume of distribution is high (1,110 L), suggesting extensive distribution into tissue. In vitro binding of [ 14 C] olodaterol to human plasma proteins is independent of concentration and is approximately 60%.
Elimination
Metabolism
Tiotropium
The extent of metabolism is small. This is evident from a urinary excretion of 74% of unchanged substance after an intravenous dose to young healthy volunteers. Tiotropium, an ester, is nonenzymatically cleaved to the alcohol N-methylscopine and dithienylglycolic acid, both not binding to muscarinic receptors.
In vitro experiments with human liver microsomes and human hepatocytes suggest that a fraction of the administered dose (74% of an intravenous dose is excreted unchanged in the urine, leaving 25% for metabolism) is metabolized by cytochrome P450-dependent oxidation and subsequent glutathione conjugation to a variety of Phase 2 metabolites. This enzymatic pathway can be inhibited by CYP450 2D6 and 3A4 inhibitors, such as quinidine, ketoconazole, and gestodene. Thus, CYP450 2D6 and 3A4 are involved in the metabolic pathway that is responsible for the elimination of a small part of the administered dose. In vitro studies using human liver microsomes showed that tiotropium in supra-therapeutic concentrations does not inhibit CYP450 1A1, 1A2, 2B6, 2C9, 2C19, 2D6, 2E1, or 3A4.
Olodaterol
Olodaterol is substantially metabolized by direct glucuronidation and by O-demethylation at the methoxy moiety followed by conjugation. Of the six metabolites identified, only the unconjugated demethylation product binds to beta 2 -receptors. This metabolite, however, is not detectable in plasma after chronic inhalation of the recommended therapeutic dose.
Cytochrome P450 isozymes CYP2C9 and CYP2C8, with negligible contribution of CYP3A4, are involved in the O-demethylation of olodaterol, while uridine diphosphate glycosyl transferase isoforms UGT2B7, UGT1A1, 1A7, and 1A9 were shown to be involved in the formation of olodaterol glucuronides.
Excretion
Tiotropium
The terminal half-life of tiotropium in COPD patients following once daily inhalation of 5 mcg tiotropium was approximately 25 hours. Total clearance was 880 mL/min after an intravenous dose in young healthy volunteers. Intravenously administered tiotropium bromide is mainly excreted unchanged in urine (74%). After inhalation of the solution by patients with COPD, urinary excretion is 18.6% (0.932 mcg) of the dose, the remainder being mainly non-absorbed drug in the gut that is eliminated via the feces. The renal clearance of tiotropium exceeds the creatinine clearance, indicating secretion into the urine. After chronic once-daily inhalation by COPD patients, pharmacokinetic steady state was reached by day 7 with no accumulation thereafter.
Olodaterol
Total clearance of olodaterol in healthy volunteers is 872 mL/min, and renal clearance is 173 mL/min. The terminal half-life following intravenous administration is 22 hours. The terminal half-life following inhalation in contrast is about 45 hours, indicating that the latter is determined by absorption rather than by elimination processes. However, the effective half-life at daily dose of 5 mcg calculated from C max from COPD patients is 7.5 hours.
Following intravenous administration of [ 14 C]-labeled olodaterol, 38% of the radioactive dose was recovered in the urine and 53% was recovered in feces. The amount of unchanged olodaterol recovered in the urine after intravenous administration was 19%. Following oral administration, only 9% of olodaterol and/or its metabolites was recovered in urine, while the major portion was recovered in feces (84%). More than 90% of the dose was excreted within 6 and 5 days following intravenous and oral administration, respectively. Following inhalation, excretion of unchanged olodaterol in urine within the dosing interval in healthy volunteers at steady state accounted for 5% to 7% of the dose.
Drug Interactions
STIOLTO RESPIMAT
Pharmacokinetic drug interaction studies with STIOLTO RESPIMAT have not been performed; however, such studies have been conducted with individual components tiotropium and olodaterol.
When tiotropium and olodaterol were administered in combination by the inhaled route, the pharmacokinetic parameters for each component were similar to those observed when each active substance was administered separately.
Tiotropium
An interaction study with tiotropium (14.4 mcg intravenous infusion over 15 minutes) and cimetidine 400 mg three times daily or ranitidine 300 mg once-daily was conducted. Concomitant administration of cimetidine with tiotropium resulted in a 20% increase in the AUC 0-4h , a 28% decrease in the renal clearance of tiotropium and no significant change in the C max and amount excreted in urine over 96 hours. Co-administration of tiotropium with ranitidine did not affect the pharmacokinetics of tiotropium.
Common concomitant medications (long-acting beta 2 -adrenergic agonists (LABA), inhaled corticosteroids (ICS)) used by patients with COPD were not found to alter the exposure to tiotropium.
Olodaterol
Drug-drug interaction studies were carried out using fluconazole as a model inhibitor of CYP 2C9 and ketoconazole as a potent P-gp (and CYP3A4, 2C8, 2C9) inhibitor.
Fluconazole : Co-administration of 400 mg fluconazole once a day for 14 days had no relevant effect on systemic exposure to olodaterol.
Ketoconazole : Co-administration of 400 mg ketoconazole once a day for 14 days increased olodaterol C max by 66% and AUC 0-1 by 68%.
Tiotropium : Co-administration of tiotropium bromide, delivered as a fixed-dose combination with olodaterol, for 21 days had no relevant effect on systemic exposure to olodaterol, and vice versa.
Specific Populations
Olodaterol
A pharmacokinetic meta-analysis showed that no dose adjustment is necessary based on the effect of age, gender, and weight on systemic exposure in COPD patients after inhalation of olodaterol.
Geriatric Patients
Tiotropium:
As expected for all predominantly renally excreted drugs, advancing age was associated with a decrease of tiotropium renal clearance (347 mL/min in COPD patients <65 years to 275 mL/min in COPD patients ≥65 years). This did not result in a corresponding increase in AUC 0-6,ss and C max,ss values.
Renal Impairment
Tiotropium
Following inhaled administration of therapeutic doses of tiotropium to steady-state to patients with COPD, mild renal impairment (creatinine clearance 60 - <90 mL/min) resulted in 23% higher AUC 0-6,ss and 17% higher C max,ss values. Moderate renal impairment (creatinine clearance 30 - <60 mL/min) resulted in 57% higher AUC 0-6,ss and 31% higher C max,ss values compared to COPD patients with normal renal function (creatinine clearance ≥90 mL/min). In COPD patients with severe renal impairment (CLCR <30 mL/min), a single intravenous administration of tiotropium bromide resulted in 94% higher AUC 0-4 and 52% higher C max compared to COPD patients with normal renal function.
Olodaterol
Olodaterol levels were increased by approximately 40% in subjects with severe renal impairment. A study in subjects with mild and moderate renal impairment was not performed.
Hepatic Impairment
Tiotropium
The effects of hepatic impairment on the pharmacokinetics of tiotropium were not studied.
Olodaterol
Subjects with mild and moderate hepatic impairment showed no changes in C max or AUC, nor did protein binding differ between mild and moderate hepatically impaired subjects and their healthy controls. A study in subjects with severe hepatic impairment was not performed.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
STIOLTO RESPIMAT
No studies of the carcinogenicity, in vitro mutagenicity, or impairment of fertility were conducted with STIOLTO RESPIMAT, however, studies are available for the individual components, tiotropium and olodaterol.
Tiotropium
No evidence of tumorigenicity was observed in a 104-week inhalation study in rats at tiotropium doses up to 59 mcg/kg/day, in an 83-week inhalation study in female mice at doses up to 145 mcg/kg/day, and in a 101-week inhalation study in male mice at doses up to 2 mcg/kg/day. These doses correspond to approximately 30, 40, and 0.5 times the recommended human daily inhalation dose (RHDID) on a mcg/m 2 basis, respectively.
Tiotropium bromide demonstrated no evidence of mutagenicity or clastogenicity in the following assays: the bacterial gene mutation assay, the V79 Chinese hamster cell mutagenesis assay, the chromosomal aberration assay in human lymphocytes in vitro , the mouse micronucleus assay in vivo , and the unscheduled DNA synthesis assay in primary rat hepatocytes in vitro .
In rats, decreases in the number of corpora lutea and the percentage of implants were noted at inhalation tiotropium doses of 78 mcg/kg/day or greater (approximately 35 times the RHDID on a mcg/m 2 basis). No such effects were observed at 9 mcg/kg/day (approximately 4 times than the RHDID on a mcg/m 2 basis). The fertility index; however, was not affected at inhalation doses up to 1,689 mg/kg/day (approximately 760 times the RHDID on a mcg/m 2 basis).
Olodaterol
Two-year inhalation studies were conducted in rats and mice to assess the carcinogenic potential of olodaterol. Lifetime treatment of female rats induced leiomyomas of the mesovarium at doses of 25.8 and 270 mcg/kg/day (approximately 18- and 198-fold, respectively, the RHDID on an AUC basis). No tumor findings were observed in male rats at doses up to 270 mcg/kg/day (approximately 230-fold the RHDID on an AUC basis). Lifetime treatment of female mice induced leiomyomas and leiomyosarcomas of the uterus at doses ≥76.9 mcg/kg/day (approximately 106-fold the RHDID on an AUC basis). No tumor findings were observed in male mice at doses up to 255 mcg/kg/day (approximately 455-fold the RHDID on an AUC basis). Increases in leiomyomas and leiomyosarcomas of the female rodent reproductive tract have been similarly demonstrated with other beta 2 -adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Olodaterol was not mutagenic in the in vitro Ames test or in the in vitro mouse lymphoma assay. Olodaterol produced increased frequency of micronuclei in rats after intravenous doses. The increased frequency of micronuclei was likely related to drug enhanced (compensatory) erythropoiesis. The mechanism for induction of micronuclei formation is likely not relevant at clinical exposures.
Olodaterol did not impair male or female fertility in rats at inhalation doses up to 3,068 mcg/kg/day (approximately 2,322 times the RHDID on an AUC basis).
CLINICAL STUDIES
The safety and efficacy of STIOLTO RESPIMAT were evaluated in a clinical development program that included three dose ranging trials, two active-controlled trials, three active- and placebo-controlled trials, and one placebo-controlled trial. The efficacy of STIOLTO RESPIMAT is based primarily on two 4-week dose-ranging trials in 592 COPD patients and two confirmatory active-controlled 52-week trials (Trials 1 and 2) in 5,162 COPD patients.
Dose-Ranging Trials
Dose selection for STIOLTO RESPIMAT was primarily based on trials for the individual components, tiotropium bromide and olodaterol.
Dose selection was also supported by two randomized, double-blind, active-controlled, 4-week trials. In one trial in 232 patients with COPD, three tiotropium doses (1.25, 2.5, and 5 mcg) were given in combination with olodaterol 5 or 10 mcg and were evaluated compared to olodaterol monotherapy. Results demonstrated improvement in trough FEV 1 for the combination when compared to olodaterol alone. The difference in trough FEV 1 for the tiotropium bromide/olodaterol doses of 1.25/5, 2.5/5, and 5/5 mcg once daily from olodaterol 5 mcg were 0.054 L (95% CI 0.016, 0.092), 0.065 L (0.027, 0.103), and 0.084 L (0.046, 0.122), respectively. In the second trial in 360 patients with COPD, three olodaterol doses (2, 5, and 10 mcg) were given in combination with tiotropium 5 mcg and were evaluated compared to tiotropium monotherapy. The difference in trough FEV 1 for the tiotropium/olodaterol doses of 5/2, 5/5, and 5/10 mcg once daily from tiotropium 5 mcg were 0.024 L (95% CI -0.029, 0.076), 0.033 L (-0.019, 0.085), and 0.057 L (0.004, 0.110), respectively. Results of these trials supported the evaluation of once-daily doses of tiotropium bromide/olodaterol 2.5/5 mcg and 5/5 mcg in the confirmatory trials.
Confirmatory Trials
A total of 5,162 COPD patients (1,029 receiving STIOLTO RESPIMAT, 1,038 receiving olodaterol 5 mcg, and 1,033 receiving tiotropium bromide 5 mcg) were studied in two confirmatory trials of STIOLTO RESPIMAT. Trials 1 and 2 were 52-week, replicate, randomized, double-blind, active controlled, parallel group trials that compared STIOLTO RESPIMAT to tiotropium 5 mcg and olodaterol 5 mcg. In these trials, all products were administered via the RESPIMAT inhaler.
The trials enrolled patients 40 years of age or older with a clinical diagnosis of COPD, a smoking history of more than 10 pack-years, and moderate to very severe pulmonary impairment (post-bronchodilator FEV 1 less than 80% predicted normal [GOLD Stage 2-4]; post-bronchodilator FEV 1 to FVC ratio of less than 70%). All treatments were administered once daily in the morning. The primary endpoints were change from baseline in FEV 1 AUC 0-3hr and trough FEV 1 after 24 weeks of treatment.
The majority of the 5162 patients were male (73%), white (71%) or Asian (25%), with a mean age of 64.0 years. Mean post-bronchodilator FEV 1 was 1.37 L (GOLD 2 [50%], GOLD 3 [39%], GOLD 4 [11%]). Mean beta 2 -agonist responsiveness was 16.6% of baseline (0.171 L). Pulmonary medications allowed as concomitant therapy included inhaled steroids [47%] and xanthines [10%].
In both Trials 1 and 2, STIOLTO RESPIMAT demonstrated significant improvements in FEV 1 AUC 0-3hr and trough FEV 1 after 24 weeks compared to tiotropium 5 mcg and olodaterol 5 mcg (Table 2). The increased bronchodilator effects of STIOLTO RESPIMAT compared to tiotropium 5 mcg and olodaterol 5 mcg were maintained throughout the 52-week treatment period. STIOLTO RESPIMAT displayed a mean increase in FEV 1 from baseline of 0.137 L (range: 0.133-0.140 L) within 5 minutes after the first dose. Patients treated with STIOLTO RESPIMAT used less rescue medication compared to patients treated with tiotropium 5 mcg and olodaterol 5 mcg.
| Trial 1 | Trial 2 | |||||
|---|---|---|---|---|---|---|
| n | Mean (L) | Difference (L) (95% CI) | n | Mean (L) | Difference (L) (95% CI) | |
| Pre-treatment baseline FEV 1 : Trial 1=1.16 L; Trial 2=1.15 L | ||||||
| p≤0.0001 for all comparisons between STIOLTO RESPIMAT and the monotherapies. | ||||||
| FEV 1 AUC 0-3hr response | ||||||
| STIOLTO RESPIMAT | 522 | 0.256 | - | 502 | 0.268 | - |
| Tiotropium 5 mcg | 526 | 0.139 | 0.117 (0.094, 0.140) | 500 | 0.165 | 0.103 (0.078, 0.127) |
| Olodaterol 5 mcg | 525 | 0.133 | 0.123 (0.100, 0.146) | 507 | 0.136 | 0.132 (0.108, 0.157) |
| Trough FEV 1 response | ||||||
| STIOLTO RESPIMAT | 521 | 0.136 | - | 497 | 0.145 | - |
| Tiotropium 5 mcg | 520 | 0.065 | 0.071 (0.047, 0.094) | 498 | 0.096 | 0.050 (0.024, 0.075) |
| Olodaterol 5 mcg | 519 | 0.054 | 0.082 (0.059, 0.106) | 503 | 0.057 | 0.088 (0.063, 0.113) |
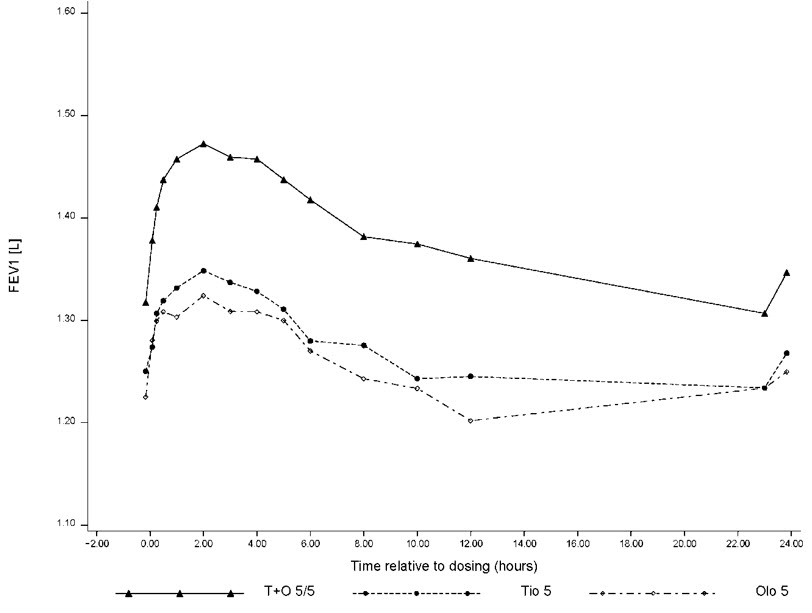
For the subset of patients (n=521) who completed extended lung function measurements up to 12 hours post-dose, STIOLTO RESPIMAT showed a significantly greater FEV 1 response compared to tiotropium 5 mcg and olodaterol 5 mcg over the full 24-hour dosing interval. Results from Trial 2 are shown in Figure 1.
Figure 1 FEV 1 profile for STIOLTO RESPIMAT, tiotropium 5 mcg and olodaterol 5 mcg over a 24-hour dosing interval after 24 weeks (12 hr PFT subset from Trial 2)
The St. George's Respiratory Questionnaire (SGRQ) was assessed in Trials 1 and 2 and in two additional 12-week placebo-controlled trials (Trials 3 and 4).
In the first 12-week trial, SGRQ responder rates at week 12 (defined as an improvement in score of 4 or more as a threshold) were 53%, 42%, and 31% for STIOLTO RESPIMAT, tiotropium 5 mcg, and placebo, respectively, with odds ratios of 1.6 (95% CI 1.1, 2.4) and 2.5 (95% CI 1.6, 3.8) for STIOLTO RESPIMAT vs. tiotropium 5 mcg and STIOLTO RESPIMAT vs. placebo, respectively. In the second 12-week trial, results were similar with odds ratios of 1.5 (95% CI 1.0, 2.3) and 2.2 (95% CI 1.5, 3.4) for STIOLTO RESPIMAT vs. tiotropium 5 mcg and STIOLTO RESPIMAT vs. placebo, respectively. For the 52-week trials similar responder rates were seen. In Trial 1, the odds ratios for STIOLTO vs. tiotropium 5 mcg and STIOLTO vs. olodaterol 5 mcg at week 24 were 1.6 (95% CI 1.2, 2.0) and 1.9 (95% CI 1.5, 2.4), respectively. The results were similar in the 52-week Trial 2, with odds ratios for STIOLTO vs. tiotropium 5 mcg and STIOLTO vs. olodaterol 5 mcg of 1.3 (95% CI 1.0, 1.7) and 1.5 (95% CI 1.1, 1.9), respectively.
Exacerbations
Tiotropium 5 mcg Trials Evaluating Exacerbations
The effect of tiotropium 5 mcg inhalation spray on exacerbations was evaluated in three 48-week randomized, double-blind, placebo-controlled clinical trials that included COPD exacerbations as the primary endpoint. Exacerbations of COPD were defined as a complex of lower respiratory events/symptoms (increase or new onset) related to the underlying COPD, with duration of three days or more, requiring a prescription of antibiotics and/or systemic steroids and/or hospitalization. In a pooled analysis of the first two trials, tiotropium 5 mcg significantly reduced the number of COPD exacerbations compared to placebo with a rate ratio of 0.78 (95% CI 0.67, 0.92). In the third trial, tiotropium 5 mcg delayed the time to first COPD exacerbation compared to placebo with a hazard ratio of 0.69 (95% CI 0.63, 0.77).
STIOLTO RESPIMAT Trial Evaluating Exacerbations
In a one-year, randomized, double-blind, active-controlled parallel group clinical trial (Trial 5), the effect of STIOLTO RESPIMAT on COPD exacerbations was compared with tiotropium 5 mcg inhalation spray. Exacerbations were defined as above. Enrolled patients (3,939 patients receiving STIOLTO RESPIMAT and 3,941 patients receiving tiotropium 5 mcg inhalation spray) had a history of COPD exacerbation in the previous 12 months. The primary endpoint was the annualized rate of moderate to severe COPD exacerbations. The majority of patients were male (71%) and Caucasian (79%). The mean age was 66 years, and mean post-bronchodilator FEV 1 percent predicted was 45%. STIOLTO RESPIMAT treatment did not demonstrate superiority to tiotropium 5 mcg inhalation spray for the primary endpoint, the annualized rate of moderate to severe COPD exacerbations, with a rate ratio of 0.93 (99% CI, 0.85-1.02, p=0.0498). The study did not reach the pre-specified significance level of 0.01.
HOW SUPPLIED/STORAGE AND HANDLING
STIOLTO RESPIMAT Inhalation Spray is supplied in a labeled carton containing one STIOLTO RESPIMAT cartridge and one STIOLTO RESPIMAT inhaler.
The STIOLTO RESPIMAT cartridge is provided as an aluminum cylinder with a tamper protection seal on the cap. The STIOLTO RESPIMAT cartridge is only intended for use with the STIOLTO RESPIMAT inhaler and should not be interchanged with any other RESPIMAT device delivered product.
The STIOLTO RESPIMAT inhaler is a cylindrical shaped plastic inhalation device with a gray colored body and a clear base. The clear base is removed to insert the cartridge. The inhaler contains a dose indicator. The light green-colored cap and the written information on the label of the gray inhaler body indicate that it is labeled for use with the STIOLTO RESPIMAT cartridge.
STIOLTO RESPIMAT Inhalation Spray is available as:
- STIOLTO RESPIMAT Inhalation Spray: 60 metered actuations (NDC 0597-0155-61)
- STIOLTO RESPIMAT Inhalation Spray: 10 metered actuations (NDC 0597-0155-70) (institutional pack)
The STIOLTO RESPIMAT cartridge has a net fill weight of at least 4 grams and when used with the STIOLTO RESPIMAT inhaler, is designed to deliver the labeled number of metered actuations after preparation for use.
When the labeled number of actuations has been dispensed from the inhaler, the RESPIMAT locking mechanism will be engaged and no more actuations can be dispensed.
After assembly, the STIOLTO RESPIMAT inhaler should be discarded at the latest 3 months after first use or when the locking mechanism is engaged, whichever comes first.
Keep out of reach of children. Do not spray into eyes.
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Avoid freezing.
Instructions for Use STIOLTO ® RESPIMAT ® (sti-OL-to- RES peh mat) (tiotropium bromide and olodaterol inhalation spray), for oral inhalation use
For Oral Inhalation Only Do not spray STIOLTO RESPIMAT into your eyes. Read these Instructions for Use before you start using STIOLTO RESPIMAT and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your medical condition or your treatment.
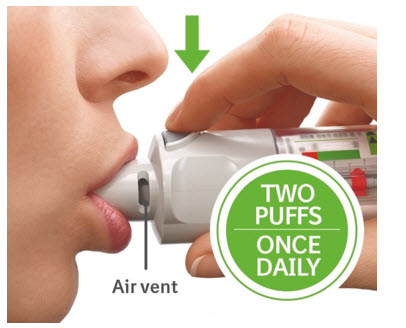
You will need to use this inhaler 1 time each day, at the same time each day. Each time you use it take 2 puffs.
Do not turn the clear base before inserting the cartridge.
How to store your STIOLTO RESPIMAT inhaler
- Store STIOLTO RESPIMAT at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze your STIOLTO RESPIMAT cartridge and inhaler.
- If STIOLTO RESPIMAT has not been used for more than 3 days, release 1 puff towards the ground.
- If STIOLTO RESPIMAT has not been used for more than 21 days, repeat steps 4 to 6 under the "Prepare for first use" until a mist is visible. Then repeat steps 4 to 6 three more times.
- Keep your STIOLTO RESPIMAT cartridge, inhaler, and all medicines out of the reach of children.
How to care for your STIOLTO RESPIMAT inhaler
Clean the mouthpiece, including the metal part inside the mouthpiece, with a damp cloth or tissue only, at least 1 time each week. Any minor discoloration in the mouthpiece does not affect your STIOLTO RESPIMAT inhaler.
When to get a new STIOLTO RESPIMAT inhaler
- The scale on your inhaler will show the number of puffs you have, if used as indicated (2 puffs 1 time each day).
- The dose indicator will show you approximately how much medicine is left.
- When the dose indicator enters the red area of the scale, it will show you approximately how many puffs are left before you need a refill or new prescription.
- When the dose indicator reaches the end of the red scale, your STIOLTO RESPIMAT is empty and automatically locks. At this point, the clear base cannot be turned any further.

- Three months after insertion of cartridge, throw away the STIOLTO RESPIMAT even if it has not been used, or when the inhaler is locked, or when it expires, whichever comes first.
Prepare for first use
1. Remove clear base
| 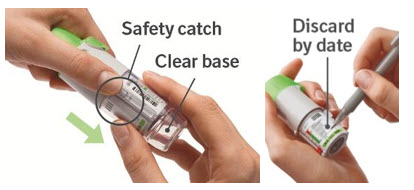 |
2. Insert cartridge
| 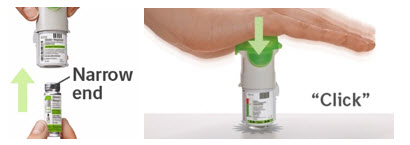 |
3. Replace clear base
| 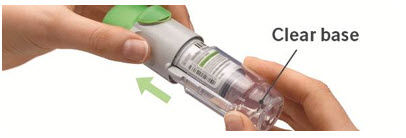 |
4. Turn
| 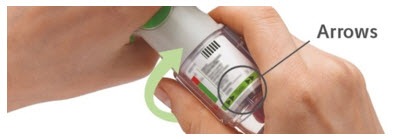 |
5. Open
|  |
6. Press
|  |
Daily use ( T O P )
T urn
|  |
O pen
|  |
P ress
|  |
Answers to Common Questions
It is difficult to insert the cartridge deep enough:
Did you accidentally turn the clear base before inserting the cartridge? Open the cap, press the dose-release button, then insert the cartridge.
Did you insert the cartridge with the wide end first? Insert the cartridge with the narrow end first.
I cannot press the dose-release button:
Did you turn the clear base? If not, turn the clear base in a continuous movement until it clicks (half a turn).
Is the dose indicator on the STIOLTO RESPIMAT pointing to 0 (zero)? The STIOLTO RESPIMAT inhaler is locked after the labeled number of puffs have been used. Prepare and use your new STIOLTO RESPIMAT inhaler.
I cannot turn the clear base:
Did you turn the clear base already? If the clear base has already been turned, follow steps "Open" and "Press" under "Daily use" to get your medicine.
Is the dose indicator on the STIOLTO RESPIMAT pointing to 0 (zero)? The STIOLTO RESPIMAT inhaler is locked after the labeled number of puffs have been used. Prepare and use your new STIOLTO RESPIMAT inhaler.
The dose indicator on the STIOLTO RESPIMAT reaches 0 (zero) too soon:
Did you use STIOLTO RESPIMAT as indicated (2 puffs 1 time each day)? STIOLTO RESPIMAT will deliver the labeled number of puffs if used at 2 puffs 1 time each day.
Did you turn the clear base before you inserted the cartridge? The dose indicator counts each turn of the clear base regardless whether a cartridge has been inserted or not.
Did you spray in the air often to check whether the STIOLTO RESPIMAT is working? After you have prepared STIOLTO RESPIMAT, no test-spraying is required if used daily.
Did you insert the cartridge into a used STIOLTO RESPIMAT? Always insert a new cartridge into a NEW STIOLTO RESPIMAT.
My STIOLTO RESPIMAT sprays automatically:
Was the cap open when you turned the clear base? Close the cap, then turn the clear base.
Did you press the dose-release button when turning the clear base? Close the cap, so the dose-release button is covered, then turn the clear base.
Did you stop when turning the clear base before it clicked? Turn the clear base in a continuous movement until it clicks (half a turn).
My STIOLTO RESPIMAT does not spray:
Did you insert a cartridge? If not, insert a cartridge.
Did you repeat Turn, Open, Press (TOP) less than 3 times after inserting the cartridge? Repeat T urn, O pen, P ress (TOP) 3 times after inserting the cartridge as shown in steps 4 to 6 under "Prepare for first use".
Is the dose indicator on the STIOLTO RESPIMAT pointing to 0 (zero)? You have used up all your medicine and the inhaler is locked.
For more information about STIOLTO RESPIMAT, including current prescribing information, a video demonstration or a Quick Start Guide on how to use STIOLTO RESPIMAT, go to www.STIOLTO.com , scan the code, or call 1-800-542-6257.

This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA
Licensed from: Boehringer Ingelheim International GmbH
SPIRIVA ® , HANDIHALER ® , STIOLTO ® and RESPIMAT ® are registered trademarks of and are used under license from Boehringer Ingelheim International GmbH
Copyright © 2025 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED
Revised: January 2025
COL10537BA092025
Mechanism of Action
STIOLTO RESPIMAT
STIOLTO RESPIMAT contains both tiotropium and olodaterol. The properties described below for the individual components apply to STIOLTO RESPIMAT. These drugs represent 2 different classes of medication (an anticholinergic and a beta-agonist) that have different effects on clinical and physiological indices.
Tiotropium
Tiotropium is a long-acting, muscarinic antagonist which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors, M 1 to M 5 . In the airways, it exhibits pharmacological effects through inhibition of M 3 -receptors at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methacholine-induced bronchoconstriction effects was dose-dependent and lasted longer than 24 hours. The bronchodilation following inhalation of tiotropium is predominantly a site-specific effect.
Olodaterol
Olodaterol is a long-acting beta 2 -adrenergic agonist (LABA). The compound exerts its pharmacological effects by binding and activation of beta 2 -adrenoceptors after topical administration by inhalation. Activation of these receptors in the airways results in a stimulation of intracellular adenyl cyclase, an enzyme that mediates the synthesis of cyclic-3', 5' adenosine monophosphate (cAMP). Elevated levels of cAMP induce bronchodilation by relaxation of airway smooth muscle cells. In vitro studies have shown that olodaterol has 241-fold greater agonist activity at beta 2 -adrenoceptors compared to beta 1 -adrenoceptors and 2,299-fold greater agonist activity compared to beta 3 -adrenoceptors. The clinical significance of these findings is unknown.
Beta-adrenoceptors are divided into three subtypes: beta 1 -adrenoceptors predominantly expressed on cardiac muscle, beta 2 -adrenoceptors predominantly expressed on airway smooth muscle, and beta 3 -adrenoceptors predominantly expressed on adipose tissue. Beta 2 -agonists cause bronchodilation. Although the beta 2 -adrenoceptor is the predominant adrenergic receptor in the airway smooth muscle, it is also present on the surface of a variety of other cells, including lung epithelial and endothelial cells and in the heart. The precise function of beta 2 -receptors in the heart is not known, but their presence raises the possibility that even highly selective beta 2 -agonists may have cardiac effects.