Tivdak prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Tivdak patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- For intravenous infusion only. Do not administer TIVDAK as an intravenous push or bolus. Do not mix with, or administer as an infusion with, other medicinal products. (2.4 )
- The recommended dose of TIVDAK is 2 mg/kg (up to a maximum of 200 mg) given as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity. (2.1 )
Recommended Dosage
The recommended dose of TIVDAK is 2 mg/kg (up to a maximum of 200 mg for patients ≥100 kg) administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity.
Premedication and Required Eye Care
Adhere to the following recommendations to reduce the risk of ocular adverse reactions [see Warnings and Precautions (5.1) ].
- Ophthalmic exam by eye care provider: Conduct an ophthalmic exam prior to initiation of TIVDAK, prior to every cycle for the first nine cycles, and as clinically indicated. The ophthalmic exam should include visual acuity, slit lamp exam of the anterior segment of the eye, and an assessment of normal eye movement.
- Topical corticosteroid eye drops: Instruct patients to administer one drop in each eye prior to each infusion and to continue to administer eye drops in each eye three times daily for 72 hours after each infusion. The initial prescription and all renewals of any corticosteroid medication should be made only after examination with a slit lamp.
- Topical ocular vasoconstrictor drops: Administer in each eye immediately prior to each infusion of TIVDAK.
- Cold packs: Use cooling eye pads during each infusion of TIVDAK.
- Topical lubricating eye drops: Instruct patients to administer for the duration of therapy and for 30 days after the last dose of TIVDAK.
- Contact lenses: Advise patients to avoid wearing contact lenses for the entire duration of therapy unless advised by their eye care provider.
Dosage Modifications for Adverse Reactions
The recommended TIVDAK dose reduction schedule is provided in Table 1 .
TIVDAK Dose Level | |
Starting dose | 2 mg/kg (up to a maximum of 200 mg for patients ≥100 kg) |
First dose reduction | 1.3 mg/kg (up to a maximum of 130 mg for patients ≥100 kg) |
Second dose reduction | 0.9 mg/kg Permanently discontinue in patients who cannot tolerate 0.9 mg/kg (up to a maximum of 90 mg for patients ≥100 kg) |
The recommended dose modifications for adverse reactions are provided in Table 2 .
Adverse Reaction | Severity | Occurrence | TIVDAK Dose Modification |
Keratitis Refer patients to an eye care provider promptly for an assessment of new or worsening ocular symptoms. | Nonconfluent superficial keratitis | Any | Monitor. |
Confluent superficial keratitis, a corneal epithelial defect, or a 3 line or more loss in best corrected visual acuity | First occurrence | Withhold dose until resolution, or improvement to nonconfluent superficial keratitis, then resume treatment at the next lower dose level. | |
Second occurrence | Permanently discontinue. | ||
Ulcerative keratitis or perforation | Any | Permanently discontinue. | |
Conjunctival or corneal scarring or symblepharon | Any scarring or symblepharon | Any | Permanently discontinue. |
Conjunctivitis and other ocular adverse reactions | Nonconfluent superficial punctate conjunctival defects, mild vasodilation | Any | Monitor. |
Confluent superficial punctate conjunctival defects, moderate to severe vasodilation | First occurrence | Withhold dose until resolution or improvement to nonconfluent superficial punctate conjunctival defects, mild vasodilation, then resume treatment at the same dose. | |
Second occurrence | Withhold dose until resolution or improvement to nonconfluent superficial punctate conjunctival defects, mild vasodilation, then resume treatment at the next lower dose level. | ||
Third occurrence | Permanently discontinue. | ||
Conjunctival ulcer, conjunctival neovascularization, or fibrovascular scarring | Any | Permanently discontinue. | |
Peripheral Neuropathy | Grade 2 | Any (initial or worsening of pre-existing condition) | Withhold dose until Grade ≤1, then resume treatment at the next lower dose level. |
Grade 3 or 4 | Any | Permanently discontinue. | |
Hemorrhage | Any grade pulmonary or CNS | Any | Permanently discontinue. |
Grade 2 in any other location | Any | Withhold until resolved, then resume treatment at the same dose. | |
Grade 3 in any other location | First occurrence | Withhold dose until resolved, then resume treatment at the same dose. | |
Second occurrence | Permanently discontinue. | ||
Grade 4 in any other location | Any | Permanently discontinue. | |
Pneumonitis | Grade 2 | Any | Withhold dose until Grade ≤1 for persistent or recurrent pneumonitis, consider resuming treatment at next lower dose level. |
Grade 3 or 4 | Any | Permanently discontinue. | |
Severe cutaneous adverse reactions (including Stevens-Johnson syndrome (SJS)) | Suspected (any grade) | Any | Immediately withhold dose and consult a specialist to confirm the diagnosis. |
Confirmed Grade 3 or 4 | Any | Permanently discontinue. |
Instructions for Preparation and Administration
- Administer TIVDAK as an intravenous infusion only.
- TIVDAK is a hazardous drug. Follow applicable special handling and disposal procedures 1 .
- DO NOT administer TIVDAK as an intravenous push or bolus.
- DO NOT mix TIVDAK with, or administer as an infusion with, other medicinal products.
Use appropriate aseptic technique for reconstitution and preparation of dosing solutions. Prior to administration, the TIVDAK vial is reconstituted with Sterile Water for Injection, USP. The reconstituted solution is subsequently diluted in an intravenous infusion bag containing one of the following: 5% Dextrose Injection USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer’s Injection, USP.
Reconstitution in Single-dose Vial
- Calculate the recommended dose based on the patient’s weight to determine the number of vials needed.
- Reconstitute each 40 mg vial with 4 mL of Sterile Water for Injection, USP, resulting in 10 mg/mL TIVDAK.
- Slowly swirl each vial until the contents are completely dissolved. Allow the reconstituted vial(s) to settle. DO NOT SHAKE THE VIAL. Do not expose to direct sunlight.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted solution should be clear to slightly opalescent, colorless to brownish-yellow and free of visible particles. Discard any vial with visible particles or discoloration.
- Based upon the calculated dose amount, the reconstituted solution from the vial(s) should be added to the infusion bag immediately. This product does not contain a preservative. If not used immediately, reconstituted vials may be stored for up to 24 hours in refrigeration at 2°C to 8°C (36 °F to 46 °F) or at room temperature up to 25°C (77°F) for up to a maximum of 8 hours prior to dilution. DO NOT FREEZE. Do not expose to direct sunlight. Discard unused vials with reconstituted solution beyond the recommended storage time.
Dilution in Infusion Bag
- Withdraw the calculated dose amount of reconstituted solution from the vial(s) and transfer into an infusion bag.
- Dilute TIVDAK with one of the following: 5% Dextrose Injection, USP, 0.9% Sodium Chloride Injection, USP or Lactated Ringer's Injection, USP. The infusion bag size should allow enough diluent to achieve a final concentration of 0.7 mg/mL to 2.4 mg/mL TIVDAK.
- Mix diluted solution by gentle inversion. DO NOT SHAKE THE BAG. Do not expose to direct sunlight.
- Visually inspect the infusion bag for any particulate matter or discoloration prior to use. The reconstituted solution should be clear to slightly opalescent, colorless to brownish-yellow and free of visible particles. Discard the infusion bag if particulate matter or discoloration is observed.
- Discard any unused portion left in the single-dose vials.
Administration
- Confirm administration of steroid and vasoconstrictor eye drops [see Dosage and Administration (2.2) ].
- Apply cold packs fully over the eyes following administration of the vasoconstrictor eye drops. Change cold packs as needed throughout infusion to ensure eye area remains cold during the entire infusion [see Dosage and Administration (2.2) ].
- Immediately administer the infusion over 30 minutes through an intravenous line containing a 0.2 µm in-line filter.
- If the infusion is not administered immediately, store the diluted TIVDAK solution in refrigeration as specified in Table 3 . Discard if storage time exceeds these limits. DO NOT FREEZE. Once removed from refrigeration, complete administration of the diluted infusion solution of TIVDAK within 4 hours (including infusion time).
Diluent Used to Prepare Solution for Infusion | Diluted TIVDAK Solution Storage |
5% Dextrose Injection, USP | Up to 24 hours at 2°C to 8°C (36°F to 46°F) |
0.9% Sodium Chloride Injection, USP | Up to 18 hours at 2°C to 8°C (36°F to 46°F) |
Lactated Ringer’s Injection, USP | Up to 12 hours at 2°C to 8°C (36°F to 46°F) |

By using PrescriberAI, you agree to the AI Terms of Use.
Tivdak prescribing information
WARNING: OCULAR TOXICITY
- TIVDAK can cause severe ocular toxicities resulting in changes in vision, including severe vision loss, and corneal ulceration. [see Warnings and Precautions (5.1) ].
- Conduct an ophthalmic exam, including an assessment of ocular symptoms, visual acuity, and slit lamp exam of the anterior segment of the eye prior to initiation of TIVDAK, prior to every cycle for the first nine cycles, and as clinically indicated. [see Dosage and Administration (2.2) and Warnings and Precautions (5.1) ] .
- Adhere to the required premedication and eye care before, during, and after infusion. [see Dosage and Administration (2.2) ].
- Withhold TIVDAK until improvement and resume, reduce the dose, or permanently discontinue, based on severity [see Dosage and Administration (2.3) , Warnings and Precautions (5.1) ].
INDICATIONS AND USAGE
TIVDAK ® is indicated for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy.
DOSAGE AND ADMINISTRATION
- For intravenous infusion only. Do not administer TIVDAK as an intravenous push or bolus. Do not mix with, or administer as an infusion with, other medicinal products. (2.4 )
- The recommended dose of TIVDAK is 2 mg/kg (up to a maximum of 200 mg) given as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity. (2.1 )
Recommended Dosage
The recommended dose of TIVDAK is 2 mg/kg (up to a maximum of 200 mg for patients ≥100 kg) administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity.
Premedication and Required Eye Care
Adhere to the following recommendations to reduce the risk of ocular adverse reactions [see Warnings and Precautions (5.1) ].
- Ophthalmic exam by eye care provider: Conduct an ophthalmic exam prior to initiation of TIVDAK, prior to every cycle for the first nine cycles, and as clinically indicated. The ophthalmic exam should include visual acuity, slit lamp exam of the anterior segment of the eye, and an assessment of normal eye movement.
- Topical corticosteroid eye drops: Instruct patients to administer one drop in each eye prior to each infusion and to continue to administer eye drops in each eye three times daily for 72 hours after each infusion. The initial prescription and all renewals of any corticosteroid medication should be made only after examination with a slit lamp.
- Topical ocular vasoconstrictor drops: Administer in each eye immediately prior to each infusion of TIVDAK.
- Cold packs: Use cooling eye pads during each infusion of TIVDAK.
- Topical lubricating eye drops: Instruct patients to administer for the duration of therapy and for 30 days after the last dose of TIVDAK.
- Contact lenses: Advise patients to avoid wearing contact lenses for the entire duration of therapy unless advised by their eye care provider.
Dosage Modifications for Adverse Reactions
The recommended TIVDAK dose reduction schedule is provided in Table 1 .
TIVDAK Dose Level | |
Starting dose | 2 mg/kg (up to a maximum of 200 mg for patients ≥100 kg) |
First dose reduction | 1.3 mg/kg (up to a maximum of 130 mg for patients ≥100 kg) |
Second dose reduction | 0.9 mg/kg Permanently discontinue in patients who cannot tolerate 0.9 mg/kg (up to a maximum of 90 mg for patients ≥100 kg) |
The recommended dose modifications for adverse reactions are provided in Table 2 .
Adverse Reaction | Severity | Occurrence | TIVDAK Dose Modification |
Keratitis Refer patients to an eye care provider promptly for an assessment of new or worsening ocular symptoms. | Nonconfluent superficial keratitis | Any | Monitor. |
Confluent superficial keratitis, a corneal epithelial defect, or a 3 line or more loss in best corrected visual acuity | First occurrence | Withhold dose until resolution, or improvement to nonconfluent superficial keratitis, then resume treatment at the next lower dose level. | |
Second occurrence | Permanently discontinue. | ||
Ulcerative keratitis or perforation | Any | Permanently discontinue. | |
Conjunctival or corneal scarring or symblepharon | Any scarring or symblepharon | Any | Permanently discontinue. |
Conjunctivitis and other ocular adverse reactions | Nonconfluent superficial punctate conjunctival defects, mild vasodilation | Any | Monitor. |
Confluent superficial punctate conjunctival defects, moderate to severe vasodilation | First occurrence | Withhold dose until resolution or improvement to nonconfluent superficial punctate conjunctival defects, mild vasodilation, then resume treatment at the same dose. | |
Second occurrence | Withhold dose until resolution or improvement to nonconfluent superficial punctate conjunctival defects, mild vasodilation, then resume treatment at the next lower dose level. If no resolution or improvement to nonconfluent superficial punctate conjunctival defects, mild vasodilation, permanently discontinue. | ||
Third occurrence | Permanently discontinue. | ||
Conjunctival ulcer, conjunctival neovascularization, or fibrovascular scarring | Any | Permanently discontinue. | |
Peripheral Neuropathy | Grade 2 | Any (initial or worsening of pre-existing condition) | Withhold dose until Grade ≤1, then resume treatment at the next lower dose level. |
Grade 3 or 4 | Any | Permanently discontinue. | |
Hemorrhage | Any grade pulmonary or CNS | Any | Permanently discontinue. |
Grade 2 in any other location | Any | Withhold until resolved, then resume treatment at the same dose. | |
Grade 3 in any other location | First occurrence | Withhold dose until resolved, then resume treatment at the same dose. | |
Second occurrence | Permanently discontinue. | ||
Grade 4 in any other location | Any | Permanently discontinue. | |
Pneumonitis | Grade 2 | Any | Withhold dose until Grade ≤1 for persistent or recurrent pneumonitis, consider resuming treatment at next lower dose level. |
Grade 3 or 4 | Any | Permanently discontinue. | |
Severe cutaneous adverse reactions (including Stevens-Johnson syndrome (SJS)) | Suspected (any grade) | Any | Immediately withhold dose and consult a specialist to confirm the diagnosis. |
Confirmed Grade 3 or 4 | Any | Permanently discontinue. |
Instructions for Preparation and Administration
- Administer TIVDAK as an intravenous infusion only.
- TIVDAK is a hazardous drug. Follow applicable special handling and disposal procedures 1 .
- DO NOT administer TIVDAK as an intravenous push or bolus.
- DO NOT mix TIVDAK with, or administer as an infusion with, other medicinal products.
Use appropriate aseptic technique for reconstitution and preparation of dosing solutions. Prior to administration, the TIVDAK vial is reconstituted with Sterile Water for Injection, USP. The reconstituted solution is subsequently diluted in an intravenous infusion bag containing one of the following: 5% Dextrose Injection USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer’s Injection, USP.
Reconstitution in Single-dose Vial
- Calculate the recommended dose based on the patient’s weight to determine the number of vials needed.
- Reconstitute each 40 mg vial with 4 mL of Sterile Water for Injection, USP, resulting in 10 mg/mL TIVDAK.
- Slowly swirl each vial until the contents are completely dissolved. Allow the reconstituted vial(s) to settle. DO NOT SHAKE THE VIAL. Do not expose to direct sunlight.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted solution should be clear to slightly opalescent, colorless to brownish-yellow and free of visible particles. Discard any vial with visible particles or discoloration.
- Based upon the calculated dose amount, the reconstituted solution from the vial(s) should be added to the infusion bag immediately. This product does not contain a preservative. If not used immediately, reconstituted vials may be stored for up to 24 hours in refrigeration at 2°C to 8°C (36 °F to 46 °F) or at room temperature up to 25°C (77°F) for up to a maximum of 8 hours prior to dilution. DO NOT FREEZE. Do not expose to direct sunlight. Discard unused vials with reconstituted solution beyond the recommended storage time.
Dilution in Infusion Bag
- Withdraw the calculated dose amount of reconstituted solution from the vial(s) and transfer into an infusion bag.
- Dilute TIVDAK with one of the following: 5% Dextrose Injection, USP, 0.9% Sodium Chloride Injection, USP or Lactated Ringer's Injection, USP. The infusion bag size should allow enough diluent to achieve a final concentration of 0.7 mg/mL to 2.4 mg/mL TIVDAK.
- Mix diluted solution by gentle inversion. DO NOT SHAKE THE BAG. Do not expose to direct sunlight.
- Visually inspect the infusion bag for any particulate matter or discoloration prior to use. The reconstituted solution should be clear to slightly opalescent, colorless to brownish-yellow and free of visible particles. Discard the infusion bag if particulate matter or discoloration is observed.
- Discard any unused portion left in the single-dose vials.
Administration
- Confirm administration of steroid and vasoconstrictor eye drops [see Dosage and Administration (2.2) ].
- Apply cold packs fully over the eyes following administration of the vasoconstrictor eye drops. Change cold packs as needed throughout infusion to ensure eye area remains cold during the entire infusion [see Dosage and Administration (2.2) ].
- Immediately administer the infusion over 30 minutes through an intravenous line containing a 0.2 µm in-line filter.
- If the infusion is not administered immediately, store the diluted TIVDAK solution in refrigeration as specified in Table 3 . Discard if storage time exceeds these limits. DO NOT FREEZE. Once removed from refrigeration, complete administration of the diluted infusion solution of TIVDAK within 4 hours (including infusion time).
Diluent Used to Prepare Solution for Infusion | Diluted TIVDAK Solution Storage Conditions (Including Infusion Time) |
5% Dextrose Injection, USP | Up to 24 hours at 2°C to 8°C (36°F to 46°F) |
0.9% Sodium Chloride Injection, USP | Up to 18 hours at 2°C to 8°C (36°F to 46°F) |
Lactated Ringer’s Injection, USP | Up to 12 hours at 2°C to 8°C (36°F to 46°F) |
DOSAGE FORMS AND STRENGTHS
For Injection: 40 mg of tisotumab vedotin-tftv as a white to off-white lyophilized cake or powder in a single-dose vial for reconstitution.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Based on the mechanism of action and findings in animals, TIVDAK can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1) ] . There are no available human data on TIVDAK use in pregnant women to inform a drug-associated risk. In an animal reproduction study, administration of the small molecule component of TIVDAK, MMAE, to pregnant rats during organogenesis caused embryo-fetal mortality and structural abnormalities at exposures below the clinical exposure at the recommended dose (see Data ) . Advise patients of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data Animal Data
No embryo-fetal development studies in animals have been performed with tisotumab vedotin-tftv. In an embryo-fetal development study in pregnant rats, administration of two intravenous doses of MMAE, the small molecule component of TIVDAK, on gestational days 6 and 13 caused embryo-fetal mortality and structural abnormalities, including protruding tongue, malrotated limbs, gastroschisis, and agnathia compared to controls at a dose of 0.2 mg/kg (approximately 0.5-fold the human area under the curve [AUC] at the recommended dose).
Lactation
Risk Summary
There are no data on the presence of tisotumab vedotin-tftv in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise lactating women not to breastfeed during treatment with TIVDAK and for 3 weeks after the last dose.
Females and Males of Reproductive Potential
TIVDAK can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1) ].
Pregnancy testing
Verify pregnancy status in females of reproductive potential prior to initiating TIVDAK treatment.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with TIVDAK and for 2 months after the last dose.
Males
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TIVDAK and for 4 months after the last dose.
Infertility
Females
Based on findings in animal studies with MMAE-containing antibody-drug conjugates (ADCs), TIVDAK may impair female fertility. The effect on fertility is reversible [see Nonclinical Toxicology (13.1) ].
Males
Based on findings from animal studies, TIVDAK may impair male fertility [see Nonclinical Toxicology (13.1) ].
Pediatric Use
Safety and effectiveness of TIVDAK in pediatric patients have not been established.
Nonclinical studies of tisotumab vedotin-tftv suggest a lack of anti-tumor activity and penetrance to brain based on limited in vivo data from tissue factor (TF)-expressing subcutaneous or orthotopic pediatric models of high‑grade gliomas/glioblastomas (HGG/GBM) and diffuse intrinsic pontine glioma (DIPG).
Geriatric Use
Of the 425 patients with cervical cancer treated with TIVDAK across clinical trials, 14% were ≥65 years of age and 2.4% were ≥75 years of age. Grade ≥3 adverse reactions occurred in 60% of patients ≥65 years and in 55% of patients <65 years. Drug was discontinued due to an adverse reaction in 25% of patients ≥65 years and in 13% of patients <65 years. Clinical studies of TIVDAK did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger patients.
Hepatic Impairment
Avoid use of TIVDAK in patients with moderate or severe hepatic impairment [aspartate aminotransferase (AST) > 3 x upper limit of normal (ULN) or total bilirubin > 1.5 × ULN] [see Clinical Pharmacology (12.3) ].
In patients with mild hepatic impairment (total bilirubin ≤ ULN and AST >ULN or total bilirubin > 1 to 1.5 × ULN and any AST), closely monitor patients for adverse reactions of TIVDAK, but no dosage adjustment in the starting dose of TIVDAK is recommended.
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
- Peripheral neuropathy: Monitor patients for new or worsening peripheral neuropathy. Withhold, reduce the dose, or permanently discontinue TIVDAK based on severity. (2.3 , 5.2 )
- Hemorrhage: Monitor patients for signs and symptoms of hemorrhage. Withhold, reduce the dose, or permanently discontinue TIVDAK based on severity. (2.3 , 5.3 )
- Pneumonitis: Severe, life-threatening, or fatal pneumonitis may occur. Withhold TIVDAK for persistent or recurrent Grade 2 pneumonitis and consider dose reduction. Permanently discontinue TIVDAK for Grade 3 or 4 pneumonitis. (2.3 , 5.4 )
- Severe Cutaneous Adverse Reactions: Severe cutaneous adverse reactions, including events of fatal or life-threatening Stevens-Johnson syndrome (SJS), can occur in patients treated with TIVDAK. Immediately withhold TIVDAK for suspected severe cutaneous adverse reactions, including SJS. Permanently discontinue TIVDAK for confirmed Grade 3 or 4 severe cutaneous adverse reactions, including SJS. (2.3 , 5.5 )
- Embryo-fetal toxicity: TIVDAK can cause fetal harm. Advise of the potential risk to a fetus and to use effective contraception. (5.6 , 8.1 , 8.3 )
Ocular Adverse Reactions
TIVDAK can cause severe ocular adverse reactions, including conjunctivitis, keratopathy (keratitis, punctate keratitis, and ulcerative keratitis), and dry eye (increased lacrimation, eye pain, eye discharge, pruritus, irritation, and foreign body sensation), that may lead to changes in vision and/or corneal ulceration.
Ocular adverse reactions occurred in 55% of patients with cervical cancer treated with TIVDAK across clinical trials. The most common ocular adverse reactions were conjunctivitis (32%), dry eye (24%), keratopathy (17%), and blepharitis (5%). Grade 3 ocular adverse reactions occurred in 3.3% of patients, including severe ulcerative keratitis in 1.2% of patients. Nine patients (2.1%) experienced ulcerative keratitis (including one with perforation requiring corneal transplantation), six (1.4%) conjunctival ulcer, four (0.9%) corneal erosion, two (0.5%) conjunctival erosion, and two (0.5%) symblepharon.
The median time to onset of the first ocular adverse reaction was 1.2 months (range, 0-17.1). Of the patients who experienced ocular events, 59% had complete resolution and 31% had partial improvement (defined as a decrease in severity by one or more grades from the worst grade) at last follow up. Ocular adverse reactions led to permanent discontinuation of TIVDAK in 6% of patients with cervical cancer.
In innovaTV 301, 8 (3.2%) patients experienced delayed ocular adverse reactions occurring more than 30 days after discontinuation of TIVDAK. These adverse reactions included 3 patients with ulcerative keratitis, and one patient (each) with keratitis, punctate keratitis and corneal erosion, blepharitis and conjunctival hyperemia, conjunctival scar, and conjunctivitis and xerophthalmia.
Refer patients to an eye care provider to conduct an ophthalmic exam prior to initiation of TIVDAK, prior to every cycle for the first nine cycles, and as clinically indicated. The exam should include visual acuity, slit lamp exam of the anterior segment of the eye, and an assessment of normal eye movement and ocular signs or symptoms which include dry or irritated eyes, eye secretions or blurry vision.
Adhere to the required premedication and eye care before, during, and after infusion to reduce the risk of ocular adverse reactions [see Dosage and Administration (2.2) ].
Monitor for ocular toxicity and promptly refer patients to an eye care provider for any new or worsening ocular signs and symptoms. Withhold, reduce, or permanently discontinue TIVDAK based on the severity or persistence of the ocular adverse reaction [see Dosage and Administration (2.3) ].
Peripheral Neuropathy
Peripheral neuropathy occurred in 39% of patients with cervical cancer treated with TIVDAK across clinical trials; 6% of patients experienced Grade 3 peripheral neuropathy. Peripheral neuropathy adverse reactions included peripheral sensory neuropathy (23%), peripheral neuropathy (5%), paresthesia (3.8%), peripheral sensorimotor neuropathy (3.3%), muscular weakness (2.8%), and peripheral motor neuropathy (2.4%). One patient with another tumor type treated with TIVDAK at the recommended dose developed Guillain-Barre syndrome.
The median time to onset of peripheral neuropathy was 2.4 months (range, 0-11.3). Of the patients who experienced peripheral neuropathy, 18% had complete resolution and 21% had partial improvement (defined as a decrease in severity by one or more grades from the worst grade) at last follow up. Peripheral neuropathy led to discontinuation of TIVDAK in 7% of patients with cervical cancer.
Monitor patients for signs and symptoms of neuropathy, such as paresthesia, tingling or a burning sensation, neuropathic pain, muscle weakness, or dysesthesia. For patients experiencing new or worsening peripheral neuropathy, withhold dose, then dose reduce, or permanently discontinue TIVDAK based on the severity of peripheral neuropathy [see Dosage and Administration (2.3) ] .
Hemorrhage
Hemorrhage occurred in 51% of patients with cervical cancer treated with TIVDAK across clinical trials. The most common all grade hemorrhage adverse reaction was epistaxis (33%). Grade 3 hemorrhage occurred in 4% of patients.
The median time to onset of hemorrhage was 0.3 months (range, 0-10.4). Of the patients who experienced hemorrhage, 71% had complete resolution and 12% had partial resolution (defined as a decrease in severity by one or more grades from the worst grade) at last follow-up.
Monitor patients for signs and symptoms of hemorrhage. For patients experiencing pulmonary or CNS hemorrhage, permanently discontinue TIVDAK. For grade ≥2 hemorrhage in any other location, withhold until bleeding has resolved, blood hemoglobin is stable, there is no bleeding diathesis that could increase the risk of continuing therapy, and there is no anatomical or pathologic condition that can increase the risk of hemorrhage recurrence. After resolution, either resume treatment or permanently discontinue TIVDAK [see Dosage and Administration (2.3) ] .
Pneumonitis
Severe, life-threatening, or fatal pneumonitis can occur in patients treated with antibody drug conjugates containing vedotin including TIVDAK. Among patients with cervical cancer treated with TIVDAK across clinical trials, 4 patients (0.9%) experienced pneumonitis, including 1 patient who had a fatal outcome.
Monitor patients for pulmonary symptoms indicative of pneumonitis. Symptoms may include hypoxia, cough, dyspnea or interstitial infiltrates on radiologic exams. Infectious, neoplastic, and other causes for such symptoms should be excluded through appropriate investigations.
Withhold TIVDAK for patients who develop persistent or recurrent Grade 2 pneumonitis and consider dose reduction. Permanently discontinue TIVDAK in all patients with Grade 3 or 4 pneumonitis [see Dosage and Administration (2.3) ] .
Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, including events of fatal or life-threatening SJS, can occur in patients treated with TIVDAK. Severe cutaneous adverse reactions occurred in 1.6% of patients with cervical cancer treated with TIVDAK across clinical trials. Grade ≥3 severe cutaneous adverse reactions occurred in 0.5% of patients, including 1 patient who had a fatal outcome.
Monitor patients for signs or symptoms of severe cutaneous adverse reactions, which include target lesions, worsening skin reactions, blistering or peeling of the skin, painful sores in mouth, nose, throat, or genital area, fever or flu-like symptoms, and swollen lymph nodes. If signs or symptoms of severe cutaneous adverse reactions occur, withhold TIVDAK until the etiology of the reaction has been determined. Early consultation with a specialist is recommended to ensure greater diagnostic accuracy and appropriate management. Permanently discontinue TIVDAK for confirmed Grade 3 or 4 severe cutaneous adverse reactions, including SJS [see Dosage and Administration (2.3) ] .
Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, TIVDAK can cause fetal harm when administered to a pregnant woman. The small molecule component of TIVDAK, MMAE, administered to rats caused adverse developmental outcomes, including embryo-fetal mortality and structural abnormalities, at exposures below those occurring clinically at the recommended dose.
Advise patients of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TIVDAK and for 2 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TIVDAK and for 4 months after the last dose [see Use in Specific Populations (8.1 , 8.3 ), and Clinical Pharmacology (12.1) ].
ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Ocular Adverse Reactions [see Boxed Warning , Warnings and Precautions (5.1) ]
- Peripheral Neuropathy [see Warnings and Precautions (5.2) ]
- Hemorrhage [see Warnings and Precautions (5.3) ]
- Pneumonitis [see Warnings and Precautions (5.4) ]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.5) ]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the WARNINGS AND PRECAUTIONS section reflect exposure to TIVDAK in 425 patients with recurrent or metastatic cervical cancer who received at least one dose of TIVDAK at 2 mg/kg intravenously every 3 weeks in innovaTV 301, innovaTV 204, innovaTV 201 (NCT02001623), innovaTV 202 (NCT02552121), innovaTV 203 (NCT03245736), and innovaTV 206 (NCT03913741). The median duration of treatment with TIVDAK was 3.7 months (range: 0.4-40.2). In this pooled safety population, the most common (≥25%) adverse reactions, including laboratory abnormalities, were hemoglobin decreased (45%), peripheral neuropathy (39%), conjunctival adverse reactions (38%), nausea (37%), fatigue (36%), aspartate aminotransferase increased (33%), epistaxis (33%), alopecia (31%), alanine aminotransferase increased (30%), and hemorrhage (28%).
The data described in this section reflect exposure to TIVDAK from innovaTV 301 and innovaTV 204.
innovaTV 301
The safety of TIVDAK was evaluated in an open-label, randomized study in patients with recurrent or metastatic cervical cancer with disease progression on or after systemic therapy [see Clinical Studies (14.1) ]. A total of 250 patients received TIVDAK 2 mg/kg every 3 weeks until disease progression or unacceptable toxicity. The median duration of treatment with TIVDAK was 3.7 months (range: 0.4-19).
Serious adverse reactions occurred in 33% of patients receiving TIVDAK. The most common (≥2%) serious adverse reactions were urinary tract infection (4.8%), small intestinal obstruction (2.4%), sepsis (2%), abdominal pain (2%), and hemorrhage (2%). Fatal adverse reactions occurred in 1.6% of patients who received TIVDAK, including acute kidney injury (0.4%), pneumonia (0.4%), sepsis (0.4%) and Stevens-Johnson syndrome (0.4%).
Adverse reactions leading to permanent discontinuation occurred in 15% of patients receiving TIVDAK; the most common (≥3%) adverse reactions leading to permanent discontinuation were peripheral neuropathy (6%) and ocular adverse reactions (6%).
Adverse reactions leading to dose interruption occurred in 39% of patients receiving TIVDAK; the most common (≥3%) adverse reactions leading to dose interruption were ocular adverse reactions (16%) and peripheral neuropathy (6%).
Adverse reactions leading to dose reduction occurred in 30% of patients receiving TIVDAK; the most common (≥3%) adverse reactions leading to dose reduction were peripheral neuropathy (10%) and ocular adverse reactions (10%). The ocular adverse reactions included conjunctival disorders (4.8%), keratopathy (4%), and dry eye (0.8%).
The most common (≥25%) adverse reactions, including laboratory abnormalities, in patients receiving TIVDAK were hemoglobin decreased, peripheral neuropathy, conjunctival adverse reactions, aspartate aminotransferase increased, nausea, alanine aminotransferase increased, fatigue, sodium decreased, epistaxis, and constipation.
Table 4 summarizes the all grade and Grade 3-4 adverse reactions from innovaTV 301.
Adverse Reaction | TIVDAK N=250 | Chemotherapy N=239 | ||
All Grades | Grade 3-4 | All Grades | Grade 3-4 | |
% | % | % | % | |
Nervous system disorders | ||||
Peripheral neuropathy Peripheral neuropathy includes peripheral sensory neuropathy, paresthesia, muscular weakness, peripheral sensorimotor neuropathy, peripheral motor neuropathy, neurotoxicity, gait disturbance, neuralgia, hypoaesthesia, neuropathy peripheral, and skin burning sensation | 38 | 6 | 4.2 | 0.4 |
Eye disorders | ||||
Conjunctival adverse reactions Conjunctival adverse reactions includes conjunctivitis, conjunctivitis bacterial, conjunctivitis viral, episcleritis, ocular hyperemia, conjunctival hemorrhage, conjunctival hyperemia, conjunctival ulcer, conjunctivitis allergic, conjunctival disorder, symblepharon, conjunctival erosion, conjunctival oedema, conjunctivochalasis, and conjunctival scar | 37 | 0 | 1.7 | 0 |
Corneal adverse reactions Corneal adverse reactions includes keratitis, punctate keratitis, corneal erosion, ulcerative keratitis, corneal degeneration, corneal opacity, and keratopathy | 21 | 3.2 | 0 | 0 |
Dry eye Dry eye includes dry eye, eye discharge, eye pruritus, eye pain, eye irritation, and lacrimation increased | 21 | 0 | 1.7 | 0 |
Gastrointestinal disorders | ||||
Nausea Nausea includes nausea and retching | 33 | 0.4 | 40 | 2.1 |
Constipation | 25 | 1.2 | 16 | 0 |
Diarrhea Diarrhea includes diarrhea and gastroenteritis | 22 | 1.6 | 15 | 1.3 |
Abdominal Pain Abdominal pain includes abdominal pain, abdominal pain upper, abdominal discomfort, and abdominal pain lower | 18 | 4 | 15 | 2.5 |
Vomiting | 18 | 1.6 | 18 | 1.3 |
General | ||||
Fatigue Fatigue includes fatigue and asthenia | 28 | 6 | 32 | 6 |
Pyrexia | 17 | 0.4 | 21 | 0.8 |
Pruritus | 10 | 0.4 | 2.9 | 0 |
Vascular disorders | ||||
Epistaxis | 26 | 0 | 2.5 | 0 |
Hemorrhage Hemorrhage includes hematuria, vaginal hemorrhage, rectal hemorrhage, hemoptysis, anal hemorrhage, hemorrhage, gastrointestinal hemorrhage, hemorrhagic shock, lower gastrointestinal hemorrhage, gingival bleeding, tumor hemorrhage, intra-abdominal hemorrhage, gastric hemorrhage, genital hemorrhage, uterine hemorrhage, urinary tract hemorrhage, hemorrhoidal hemorrhage | 21 | 2 | 11 | 2.5 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 24 | 0.8 | 18 | 0.4 |
Decreased weight | 10 | 0.4 | 5 | 0 |
Skin and subcutaneous tissue disorders | ||||
Alopecia | 24 | 0 | 2.9 | 0 |
Rash Rash includes rash maculo-papular, eczema, rash macular, rash pustular, dermatitis acneiform, erythema, rash, urticaria, dermatitis, and rash erythematous | 17 | 1.6 | 16 | 1.3 |
Infections | ||||
Urinary tract infection Urinary tract infection includes urinary tract infection, pyelonephritis acute, cystitis, and urinary tract infection bacterial | 16 | 5 | 18 | 8 |
Clinically relevant adverse reactions in <10% of patients who received TIVDAK in innovaTV 301 include periorbital adverse reactions (9%) and intestinal obstruction (4.4%, including small bowel, large bowel, and malignant gastrointestinal obstruction).
Table 5 summarizes the laboratory abnormalities in innovaTV 301.
TIVDAK The denominator used to calculate the rate varied from 206 to 244 based on the number of patients with a baseline value and at least one post-treatment value. | Chemotherapy | |||
All Grades (%) | Grades 3 or 4 (%) | All Grades (%) | Grades 3-4 (%) | |
Hematology | ||||
Hemoglobin decreased | 41 | 7 | 70 | 23 |
Neutrophils decreased | 16 | 3.3 | 39 | 17 |
Chemistry | ||||
Aspartate aminotransferase increased | 34 | 2.5 | 32 | 0.4 |
Alanine aminotransferase increased | 30 | 3.3 | 35 | 2.6 |
Sodium decreased | 27 | 0.4 | 27 | 0.4 |
Creatinine increased | 23 | 2 | 26 | 2.2 |
Coagulation | ||||
Activated partial thromboplastin time prolonged | 16 | 1.9 | 17 | 0.5 |
innovaTV 204
The safety of TIVDAK was evaluated in a single arm study in patients (n=101) with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy [see Clinical Studies (14.1) ]. Patients received TIVDAK 2 mg/kg every 3 weeks until disease progression or unacceptable toxicity. The median duration of treatment was 4.2 months (range: 0.7-16).
Serious adverse reactions occurred in 43% of patients. The most common (≥3%) serious adverse reactions were ileus (6%), hemorrhage (5%), pneumonia (4%), peripheral neuropathy, sepsis, constipation, and pyrexia (each 3%). Fatal adverse reactions occurred in 4% of patients who received TIVDAK, including septic shock (1%), pneumonitis (1%), sudden death (1%), and multisystem organ failure (1%).
Adverse reactions leading to permanent discontinuation occurred in 13% of patients receiving TIVDAK; the most common (≥3%) adverse reactions leading to permanent discontinuation were peripheral neuropathy (5%) and corneal adverse reactions (4%).
Adverse reactions leading to dose interruption occurred in 47% of patients; the most (≥3%) common adverse reactions leading to dose interruption were peripheral neuropathy (8%), conjunctival adverse reactions (4%), and hemorrhage (4%).
Adverse reactions leading to dose reduction occurred in 23% of patients; the most common (≥3%) adverse reactions leading to dose reduction were conjunctival adverse reactions (9%) and corneal adverse reactions (8%).
The most common (≥25%) adverse reactions, including laboratory abnormalities, were hemoglobin decreased, fatigue, lymphocytes decreased, nausea, peripheral neuropathy, alopecia, epistaxis, conjunctival adverse reactions, hemorrhage, leukocytes decreased, creatinine increased, dry eye, prothrombin international normalized ratio increased, activated partial thromboplastin time prolonged, diarrhea, and rash.
Table 6 summarizes the all grade and Grade 3-4 adverse reactions from innovaTV 204.
Adverse Reaction | TIVDAK N=101 | |
All Grades % | Grade 3-4 % | |
General | ||
Fatigue Fatigue includes fatigue and asthenia | 50 | 7 |
Pyrexia | 16 | 1 |
Pruritus | 13 | 1 |
Gastrointestinal disorders | ||
Nausea Nausea includes nausea and retching | 41 | 0 |
Diarrhea Diarrhea includes diarrhea, gastroenteritis, and colitis | 25 | 2 |
Constipation | 23 | 2 |
Abdominal pain Abdominal pain includes abdominal pain, abdominal pain upper, abdominal pain lower, abdominal distention and abdominal discomfort | 23 | 1 |
Vomiting | 17 | 2 |
Nervous system disorders | ||
Peripheral neuropathy Peripheral neuropathy includes neuropathy peripheral, peripheral sensorimotor neuropathy, polyneuropathy, peripheral sensory neuropathy, paresthesia, hypoesthesia, burning sensation, neuralgia, sensory loss, peripheral motor neuropathy, muscular weakness, gait disturbance, and hyperesthesia | 39 | 7 |
Skin and subcutaneous tissue disorders | ||
Alopecia | 39 | 0 |
Rash Rash includes rash, rash maculo-papular, rash macular, dermatitis acneiform, dermatitis allergic, and erythema | 25 | 0 |
Vascular disorders | ||
Epistaxis | 39 | 0 |
Hemorrhage Hemorrhage includes vaginal hemorrhage, hematuria, rectal hemorrhage, cystitis hemorrhagic, lower gastrointestinal hemorrhage, urinary bladder hemorrhage, hematochezia, anal hemorrhage, gingival bleeding, post procedural hemorrhage, radiation associated with hemorrhage, metrorrhagia, large intestinal hemorrhage, paranasal sinus hemorrhage, and hemoptysis | 32 | 6 |
Eye disorders | ||
Conjunctival adverse reactions Conjunctival adverse reactions includes conjunctivitis, conjunctival abrasion, conjunctival erosion, conjunctival hyperemia, conjunctival scar, noninfective conjunctivitis, ocular hyperemia, and conjunctival hemorrhage | 37 | 0 |
Dry eye Dry eye includes dry eye and lacrimation increased | 29 | 0 |
Corneal adverse reactions Corneal adverse reactions includes keratitis, punctate keratitis, ulcerative keratitis, corneal erosion, corneal scar, keratopathy, and corneal bleeding | 21 | 3 |
Periorbital adverse reactions Periorbital adverse reactions includes blepharitis, meibomianitis, eye pruritus, entropion, trichiasis, chalazion, and meibomian gland dysfunction | 16 | 0 |
Musculoskeletal and connective tissue disorders | ||
Myalgia Myalgia includes myalgia, musculoskeletal discomfort, and musculoskeletal pain | 21 | 0 |
Arthralgia | 16 | 0 |
Pain in extremity Pain in extremity includes pain in extremity and limb discomfort | 13 | 1 |
Metabolism and nutrition disorders | ||
Decreased appetite | 16 | 1 |
Infections | ||
Urinary tract infection Urinary tract infection includes urinary tract infection, urinary tract infection bacterial, and cystitis | 14 | 2 |
Investigations | ||
Weight decreased | 12 | 0 |
Clinically relevant adverse reactions in <10% of patients who received TIVDAK in innovaTV 204 included venous thrombosis (3%), pulmonary embolism (3%), and pneumonitis (2%).
Table 7 summarizes the laboratory abnormalities in innovaTV 204.
Laboratory Abnormality | TIVDAK The denominator used to calculate the rate varied from 96 to 101 based on the number of patients with a baseline value and at least one post-treatment value. | |
All Grades (%) | Grade 3 or 4 (%) | |
Hematology | ||
Hemoglobin decreased | 52 | 7 |
Neutrophils decreased | 21 | 3 |
Chemistry | ||
Creatinine increased | 29 | 4.1 |
Alanine aminotransferase increased | 24 | 0 |
Aspartate aminotransferase increased | 18 | 0 |
Sodium decreased | 20 | 0 |
Alkaline phosphatase increased | 17 | 0 |
Creatinine kinase increased | 16 | 2.1 |
Magnesium decreased | 17 | 2.1 |
Coagulation | ||
Prothrombin international normalized ratio increased | 26 | 0 |
Activated partial thromboplastin time prolonged | 26 | 2 |
DRUG INTERACTIONS
Strong CYP3A4 Inhibitors: Closely monitor for TIVDAK adverse reactions. (7.1 )
Effects of Other Drugs on TIVDAK
Strong CYP3A4 Inhibitors MMAE is a CYP3A4 substrate. Concomitant use of TIVDAK with strong CYP3A4 inhibitors may increase unconjugated MMAE exposure [see Clinical Pharmacology (12.3) ] , which may increase the risk of TIVDAK adverse reactions. Closely monitor patients for adverse reactions of TIVDAK when used concomitantly with strong CYP3A4 inhibitors.
DESCRIPTION
Tisotumab vedotin-tftv is a Tissue Factor (TF) directed antibody drug conjugate (ADC) comprised of a human anti-TF IgG1-kappa antibody conjugated to the microtubule-disrupting agent monomethyl auristatin E (MMAE) via a protease-cleavable vc (valine-citrulline) linker. The monoclonal antibody is produced in a mammalian cell line (Chinese hamster ovary). MMAE and the linker are produced by chemical synthesis. Each monoclonal antibody molecule carries an average of 4 MMAE molecules. Tisotumab vedotin-tftv has an approximate molecular weight of 153 kDa. The chemical structure is as follows:
Figure 1. Structural Formula
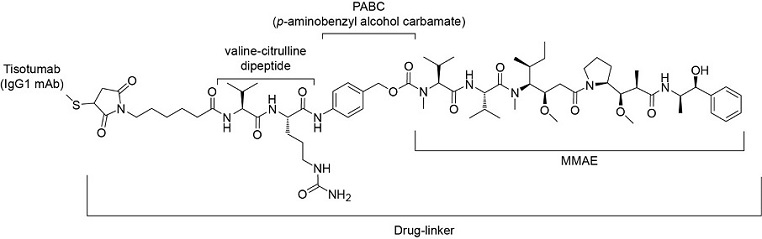
TIVDAK (tisotumab vedotin-tftv) for injection, is provided as a sterile, preservative-free, white to off-white lyophilized cake or powder in a single-dose vial for infusion after dilution. Following reconstitution with 4 mL of Sterile Water for Injection, a clear to slightly opalescent, colorless to brownish-yellow solution containing 10 mg/mL tisotumab vedotin-tftv is produced [see Dosage and Administration (2.3) ]. Each mL of reconstituted solution contains 10 mg of tisotumab vedotin-tftv, d-mannitol (30 mg), l-histidine (2.11 mg), l-histidine monohydrochloride (3.44 mg), and sucrose (30 mg), at pH 6.0.
CLINICAL PHARMACOLOGY
Mechanism of Action
Tisotumab vedotin-tftv is a tissue factor (TF)-directed antibody drug conjugate (ADC). The antibody is a human IgG1 directed against cell surface TF. TF is the primary initiator of the extrinsic blood coagulation cascade. The small molecule, MMAE, is a microtubule-disrupting agent, attached to the antibody via a protease-cleavable linker. Nonclinical data suggests that the anticancer activity of tisotumab vedotin-tftv is due to the binding of the ADC to TF expressing cancer cells, followed by internalization of the ADC-TF complex, and release of MMAE via proteolytic cleavage. MMAE disrupts the microtubule network of actively dividing cells, leading to cell cycle arrest and apoptotic cell death. In vitro, tisotumab vedotin-tftv also mediates antibody-dependent cellular phagocytosis and antibody-dependent cellular cytotoxicity.
Pharmacodynamics
Tisotumab vedotin-tftv exposure-response relationships and the time course of pharmacodynamics response have not been fully characterized.
Cardiac Electrophysiology
At the recommended dose, tisotumab vedotin-tftv had no large mean effect on QTc prolongation (>20 msec).
Pharmacokinetics
Table 8 summarizes the exposure parameters of tisotumab vedotin-tftv and unconjugated MMAE (the cytotoxic component of tisotumab vedotin-tftv) following administration of one 3-week cycle of tisotumab vedotin-tftv 2 mg/kg to patients. Tisotumab vedotin-tftv concentrations peaked near the end of the infusion, while unconjugated MMAE concentrations peaked approximately 2 to 3 days after tisotumab vedotin-tftv dosing. Tisotumab vedotin-tftv C max increased proportionally, while AUC0-last increased in a more than dose-proportional manner, after a single dose ranging from 0.3–2.2 mg/kg (0.15 to 1.1 times the approved recommended dose). There was no accumulation of tisotumab vedotin-tftv and unconjugated MMAE. Steady-state concentrations of tisotumab vedotin-tftv and unconjugated MMAE were reached after 1 treatment cycle.
| C max =maximum concentration, AUC = area under the concentration-time curve from time 0 to 21 days (3 weeks) | ||
Tisotumab Vedotin-tftv Mean (± SD) | Unconjugated MMAE Mean (± SD) | |
C max | 40.8 (8.12) μg/mL | 5.91 (4.2) ng/mL |
AUC | 57.5 (13.4) day•μg/mL | 50 (35.8) day•ng/mL |
Distribution
The tisotumab vedotin-tftv steady state volume of distribution is 7.83 (%CV: 19.1) L. Plasma protein binding of MMAE ranged from 68% to 82%, in vitro.
Elimination
The median terminal half-life of tisotumab vedotin-tftv and unconjugated MMAE is 4.04 (range: 2.26-7.25) days and 2.56 (range: 1.81-4.10) days, respectively. The linear clearance of tisotumab vedotin-tftv and unconjugated MMAE was 1.54 (%CV: 28.8) L/day and 45.9 (%CV: 61.1) L/day, respectively. Elimination of MMAE appeared to be limited by its rate of release from tisotumab vedotin-tftv.
Metabolism
Tisotumab vedotin-tftv is expected to undergo catabolism to small peptides, amino acids, unconjugated MMAE, and unconjugated MMAE-related catabolites. Tisotumab vedotin-tftv releases unconjugated MMAE via proteolytic cleavage, and unconjugated MMAE is primarily metabolized by CYP3A4 in vitro.
Excretion
The excretion of tisotumab vedotin-tftv is not fully characterized. Following a single-dose of another ADC that contains MMAE, 17% of the total MMAE administered was recovered in feces and 6% in urine over a 1-week period, primarily as unchanged drug. A similar excretion profile of MMAE is expected after tisotumab vedotin-tftv administration.
Specific Populations
No clinically significant differences in the pharmacokinetics of tisotumab vedotin-tftv were observed based on age (21 to 81 years), sex, race (white vs non-white) or ethnicity (Hispanic or Latino vs non-Hispanic or non-Latino). No clinically significant differences in exposures of tisotumab vedotin-tftv and unconjugated MMAE were observed in patients with mild to moderate renal impairment (CLcr 30 to < 90 mL/min using the Cockcroft-Gault equation) compared to patients with normal renal function. The effect of severe renal impairment (CLcr 15 to < 30 mL/min) or end-stage renal disease with or without dialysis on pharmacokinetics of tisotumab vedotin-tftv and unconjugated MMAE is unknown.
Patients with Hepatic Impairment
Unconjugated MMAE exposures were 37% higher, but there were no clinically significant differences in exposures of tisotumab vedotin-tftv in patients with mild hepatic impairment compared to patients with normal hepatic function. The effect of moderate or severe hepatic impairment or liver transplantation on the pharmacokinetics of tisotumab vedotin-tftv or unconjugated MMAE is unknown.
Drug Interaction Studies
Clinical Studies
No clinical studies evaluating the drug-drug interaction potential of tisotumab vedotin-tftv have been conducted. To characterize the drug-drug interaction potential of unconjugated MMAE, clinical studies with another ADC that contains MMAE are described below, and similar effects on tisotumab vedotin-tftv and unconjugated MMAE exposures are expected with concomitant use of TIVDAK.
There were no clinically significant differences in midazolam (sensitive CYP3A4 substrate) pharmacokinetics when used concomitantly with another ADC that contains MMAE.
Strong CYP3A4 Inhibitors: Ketoconazole (strong CYP3A4 inhibitor) used concomitantly with another ADC that contains MMAE increased unconjugated MMAE C max by 25% and AUC by 34%, with no change in ADC exposure.
Strong CYP3A4 Inducers: Rifampin (strong CYP3A4 inducer) used concomitantly with another ADC that contains MMAE decreased unconjugated MMAE C max by 44% and AUC by 46%, with no change in ADC exposure.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: MMAE does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2D6. MMAE did not induce any major CYP450 enzymes in human hepatocytes.
Transporter Systems: MMAE is a substrate of P-glycoprotein (P-gp), but not an inhibitor of P-gp.
Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of TIVDAK or of other tisotumab vedotin products.
In patients with recurrent or metastatic cervical cancer who were treated with TIVDAK 2 mg/kg every 3 weeks for up to 19 months (innovaTV 301) and 40 months (innovaTV 204) , the incidence of anti-tisotumab vedotin-tftv antibody formation was 5.7% (12/211) and 5.4% (5/93), respectively. The incidence of neutralizing anti-tisotumab vedotin-tftv antibody formation was 0% (0/211) in innovaTV 301 using cell-based assay, and the incidence was 2.2% (2/93) in innovaTV 204 using Enzyme-linked Immunosorbent Assay (ELISA)-based assay. Given the low number of patients who developed anti-tisotumab vedotin-tftv antibodies, no conclusions can be drawn concerning a potential effect of immunogenicity on pharmacokinetics, efficacy or safety.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies in animals have not been performed with tisotumab vedotin-tftv or MMAE.
MMAE was positive for genotoxicity in the in vivo rat bone marrow micronucleus study through an aneugenic mechanism. MMAE was not mutagenic in the bacterial reverse mutation (Ames) assay or the L5178 TK +/- mouse lymphoma forward mutation assay.
Fertility studies with tisotumab vedotin-tftv or MMAE have not been conducted. However, results of a repeat-dose toxicity study in monkeys indicate the potential for tisotumab vedotin-tftv to impair male reproductive function and fertility.
In a repeat-dose toxicology study conducted in monkeys for 13 weeks, doses ≥1 mg/kg tisotumab vedotin-tftv (≥0.6 times the human exposure [AUC] at the recommended dose) resulted in decreased testicular size and seminiferous tubule atrophy, reduction or absence in sperm count, and decreased sperm motility. Findings of sperm absence and decreased motility did not reverse by the end of the recovery period at doses ≥3 mg/kg (≥1.7 times the human exposure [AUC] at the recommended dose).
MMAE-containing ADCs have been associated with adverse ovarian effects when administered to sexually immature animals. Adverse effects included decrease in, or absence of, secondary and tertiary ovarian follicles after weekly administration to cynomolgus monkeys in studies of 4-week duration. These effects showed a trend towards recovery 6 weeks after the end of dosing; no changes were observed in primordial follicles.
CLINICAL STUDIES
Recurrent or Metastatic Cervical Cancer
innovaTV 301
The efficacy of TIVDAK was evaluated in innovaTV 301 (NCT04697628), an open-label, active-controlled, multicenter, randomized trial that enrolled 502 patients with recurrent or metastatic cervical cancer who had received one or two prior systemic therapy regimens in the recurrent or metastatic setting, including chemotherapy with or without bevacizumab and/or an anti-PD-(L)1 agent. Patients were excluded if they had active ocular surface disease, any prior episode of cicatricial conjunctivitis or ocular SJS, Grade ≥2 peripheral neuropathy, or clinically significant bleeding issues or risks.
Patients were randomized (1:1) to receive either TIVDAK 2 mg/kg intravenously every 3 weeks (n=253) or investigator’s choice of chemotherapy (n=249) consisting of topotecan, vinorelbine, gemcitabine, irinotecan or pemetrexed, until unacceptable toxicity or disease progression. Randomization was stratified by prior treatment with bevacizumab (yes or no), prior anti-PD-(L)1 therapy (yes or no), region (US, Europe, or Other), and ECOG performance status (0 or 1).
Patients were treated until disease progression or unacceptable toxicity. Tumor response assessments were performed every 6 weeks for the first 30 weeks and every 12 weeks thereafter.
The major efficacy outcome measure was overall survival (OS). Additional efficacy outcome measures were progression free survival (PFS) and confirmed objective response rate (ORR) as assessed by investigator using RECIST v1.1.
The median age was 50 years (range: 26 to 80); 49% were White, 36% were Asian, 10% were not reported, 3% were American Indian or Alaskan Native, and 2% were Black; 20% were Hispanic/Latino; and baseline ECOG performance status was 0 (54%) or 1 (46%). Sixty-three percent of patients had squamous cell carcinoma, 32% had adenocarcinoma, and 5% had adenosquamous histology. Ninety percent of patients had extrapelvic disease; 61% of patients had received 1 prior line of systemic therapy, and 38% had 2 prior lines of systemic therapy. All patients received prior chemotherapy; 64% received prior bevacizumab and 27% received prior anti-PD-1 or anti-PD-L1 therapy. Patients on the control arm received gemcitabine (44%), pemetrexed (32%), topotecan (8%), vinorelbine (7%), or irinotecan (6%).
Statistically significant improvements in OS, PFS, and ORR were demonstrated for TIVDAK compared with chemotherapy.
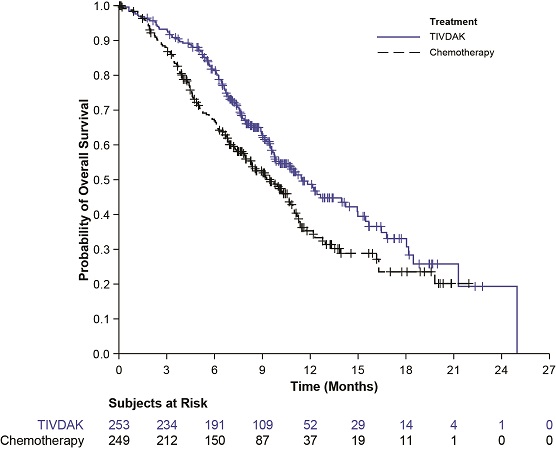
Table 9 and Figure 2 summarize the efficacy results from innovaTV 301.
| CI: confidence interval | ||
Endpoint | TIVDAK N=253 | Chemotherapy N=249 |
Overall Survival | ||
Number (%) of patients with events | 123 (48.6) | 140 (56.2) |
Median in months (95% CI) | 11.5 (9.8, 14.9) | 9.5 (7.9, 10.7) |
Hazard ratio (95% CI) | 0.70 (0.54, 0.89) | |
p-value | 0.0038 Based on a stratified log-rank test. The threshold for statistical significance is 0.0226 (2-sided) | |
Progression Free Survival | ||
Number (%) of patients with events | 198 (78.3) | 194 (77.9) |
Median in months (95% CI) | 4.2 (4.0, 4.4) | 2.9 (2.6, 3.1) |
Hazard ratio (95% CI) | 0.67 (0.54, 0.82) | |
p-value | <0.0001 Based on a stratified log-rank test. The threshold for statistical significance is 0.0453 (2-sided) | |
Confirmed Objective Response Rate (CR + PR) | ||
ORR (%) (95% CI) | 17.8 (13.3, 23.1) | 5.2 (2.8, 8.8) |
p-value | <0.0001 Based on CMH test. The threshold for statistical significance is 0.05 (2-sided) | |
Complete response rate (%) | 2.4 | 0 |
Partial response rate (%) | 15.4 | 5.2 |
Figure 2. Kaplan Meier Plot of Overall Survival
innovaTV 204
The efficacy of TIVDAK was evaluated in innovaTV 204 (NCT03438396), an open-label, multicenter, single-arm trial that treated 101 patients with recurrent or metastatic cervical cancer who had received no more than two prior systemic regimens in the recurrent or metastatic setting, including at least one prior platinum-based chemotherapy regimen. Patients were excluded if they had active ocular surface disease, any prior episode of cicatricial conjunctivitis or ocular SJS, Grade ≥2 peripheral neuropathy or known coagulation defects leading to an increased risk of bleeding.
Patients received TIVDAK 2 mg/kg intravenously every 3 weeks until disease progression or unacceptable toxicity. Tumor response assessments were performed every 6 weeks for the first 30 weeks and every 12 weeks thereafter.
The median age was 50 years (range: 31 to 78); 95% were White, 2% were Asian, and 1% were Black. Six percent of patients were Hispanic or Latino. Sixty-eight percent of patients had squamous cell carcinoma, 27% had adenocarcinoma, and 5% had adenosquamous histology. ECOG performance status was 0 (58%) or 1 (42%). Seventy percent of patients had received 1 prior line of systemic therapy, and 30% had 2 prior lines of systemic therapy. Sixty-nine percent of patients previously received bevacizumab as part of their prior systemic therapy. Sixty-three percent received bevacizumab in combination with chemotherapy (paclitaxel and cisplatin or carboplatin, or paclitaxel and topotecan) as first-line therapy.
The major efficacy outcome measures were confirmed objective response rate (ORR) as assessed by an independent review committee (IRC) using RECIST v1.1 criteria and duration of response (DOR).
Efficacy results are presented in Table 10 .
| CI: confidence interval NR: not reached | |
Endpoint | N=101 |
Confirmed ORR (95% CI) | 24% (15.9, 33.3) |
Complete response rate | 7% |
Partial response rate | 17% |
Duration of Response | |
Median Duration of Response, months Based on patients (n=24) with a response by IRC (95% CI) | 8.3 (4.2, NR) |
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
TIVDAK (tisotumab vedotin-tftv) is supplied as a white to off-white lyophilized cake or powder in a 40 mg single-dose vial for reconstitution. TIVDAK vials are available in the following packages:
- Carton of one 40 mg single-dose vial [NDC 51144-003-01]
Storage
Store TIVDAK vials refrigerated at 2ºC to 8ºC (36ºF to 46ºF) in the original carton to protect from light. Do not freeze. Do not shake.
Special Handling
TIVDAK is a hazardous drug. Follow special handling and disposal procedures. 1
Mechanism of Action
Tisotumab vedotin-tftv is a tissue factor (TF)-directed antibody drug conjugate (ADC). The antibody is a human IgG1 directed against cell surface TF. TF is the primary initiator of the extrinsic blood coagulation cascade. The small molecule, MMAE, is a microtubule-disrupting agent, attached to the antibody via a protease-cleavable linker. Nonclinical data suggests that the anticancer activity of tisotumab vedotin-tftv is due to the binding of the ADC to TF expressing cancer cells, followed by internalization of the ADC-TF complex, and release of MMAE via proteolytic cleavage. MMAE disrupts the microtubule network of actively dividing cells, leading to cell cycle arrest and apoptotic cell death. In vitro, tisotumab vedotin-tftv also mediates antibody-dependent cellular phagocytosis and antibody-dependent cellular cytotoxicity.