Trodelvy prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Patient education
Administration guides
Patient education materials
Patient support program
Dosing resources
Clinical information
Insurance resources
Prior authorization & coverage support
Reimbursement information
Financial assistance & copay programs
Legal resources
Other resources
Dosage & administration
DOSAGE AND ADMINISTRATION
- Do NOT substitute TRODELVY for or use with other drugs containing irinotecan or its active metabolite SN-38. (2.1 )
- For intravenous infusion only. Do not administer as an intravenous push or bolus.
- The recommended dose is 10 mg/kg once weekly on Days 1 and 8 of continuous 21-day treatment cycles until disease progression or unacceptable toxicity. (2.2 )
- Premedication for prevention of infusion reactions and prevention of chemotherapy-induced nausea and vomiting is recommended. (2.2 )
- Primary prophylaxis with G-CSF is recommended starting in the first cycle in all patients at increased risk of febrile neutropenia. (2.2 )
- Monitor patients during the infusion and for at least 30 minutes after completion of infusion. Treatment interruption and/or dose reduction may be needed to manage adverse reactions. (2.2 )
- See Full Prescribing Information for preparation and administration instructions. (2.4 )
Important Use Information
Do NOT substitute TRODELVY for or use with other drugs containing irinotecan or its active metabolite SN-38.
Recommended Dosage
The recommended dosage of TRODELVY is 10 mg/kg administered as an intravenous infusion once weekly on Days 1 and 8 of 21-day treatment cycles. Continue treatment until disease progression or unacceptable toxicity. Do not administer TRODELVY at doses greater than 10 mg/kg.
Administer TRODELVY as an intravenous infusion only. Do not administer as an intravenous push or bolus.
First infusion: Administer infusion over 3 hours. Observe patients during the infusion and for at least 30 minutes following the initial dose, for signs or symptoms of infusion-related reactions [see Warning and Precautions (5.3) ] .
Subsequent infusions: Administer infusion over 1 to 2 hours if prior infusions were tolerated. Observe patients during the infusion and for at least 30 minutes after infusion.
Premedication
Prior to each dose of TRODELVY, premedication for prevention of infusion reactions and prevention of chemotherapy-induced nausea and vomiting (CINV) is recommended.
- Premedicate with antipyretics, H1 and H2 blockers prior to infusion, and corticosteroids may be used for patients who had prior infusion reactions.
- Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK 1 receptor antagonist, as well as other drugs as indicated).
Prophylaxis for Neutropenia
Primary prophylaxis with granulocyte colony-stimulating factor (G-CSF) is recommended starting in the first cycle for all patients at increased risk of febrile neutropenia [see Warnings and Precautions (5.1) ] .
Dose Modifications for Adverse Reactions
Management of adverse reactions may require temporary interruption, dose reduction, or treatment discontinuation of TRODELVY as described in Tables 1 and 2. Do not re-escalate the TRODELVY dose after a dose reduction for adverse reactions has been made.
| Dose Level | Dosage and Schedule |
|---|---|
| Recommended starting dose | 10 mg/kg once weekly on Days 1 and 8 of 21-day treatment cycles |
| First dose reduction | Reduce to 7.5 mg/kg |
| Second dose reduction | Reduce to 5 mg/kg |
| Requirement for further dose reduction | Permanently discontinue TRODELVY |
The recommended dosage modifications for adverse reactions are provided in Table 2.
| Adverse reactions | Severity | Dose Modification |
|---|---|---|
| Neutropenia [see Warnings and Precautions (5.1) ] |
|
|
| Nausea/Vomiting/ Diarrhea [see Warnings and Precautions (5.2 , 5.4) ] |
|
|
| Infusion-Related Reaction [see Warnings and Precautions (5.3) ] |
|
|
|
| |
| Other Toxicities | Other Grade 3–4 toxicities of any duration despite optimal medical management |
|
Preparation and Administration
Reconstitution
- TRODELVY is a hazardous drug.
- Follow applicable special handling and disposal procedures 1 .
- Calculate the required dose (mg) of TRODELVY based on the patient's current body weight [see Dosage and Administration (2.2) ] .
- Using a sterile syringe, slowly inject 20 mL of 0.9% Sodium Chloride Injection, USP, into each 180 mg TRODELVY vial. Each vial contains overfill to compensate for liquid loss during preparation and after reconstitution, the total resulting volume delivers a concentration of 10 mg/mL .
- Gently swirl vials and allow to dissolve for up to 15 minutes. Do not shake. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be free of visible particulates, clear and yellow. Do not use the reconstituted solution if it is cloudy or discolored.
- Use immediately to prepare a diluted TRODELVY infusion solution.
Dilution
- Calculate the required amount of the reconstituted TRODELVY solution needed to obtain the appropriate dose according to the patient's body weight.
- Determine the final volume of the infusion solution to deliver the appropriate dose at a TRODELVY concentration range of 1.1 mg/mL to 3.4 mg/mL.
- Use 0.9% Sodium Chloride Injection, USP only since the stability of the reconstituted TRODELVY solution has not been determined with other infusion-based solutions. Use a polyvinyl chloride, polypropylene/polyethylene, polyolefin, or ethylene vinyl acetate infusion bag.
- Withdraw and discard the volume of 0.9% Sodium Chloride Injection, USP from the final infusion bag that is necessary to achieve the indicated TRODELVY concentration following the addition of the calculated amount of reconstituted TRODELVY solution.
- Withdraw the calculated amount of the reconstituted TRODELVY solution from the vial(s) using a syringe. Discard any unused portion remaining in the vial(s).
- To minimize foaming, slowly inject the calculated amount of reconstituted TRODELVY solution into the infusion bag. Do not shake the contents.
- If not used immediately, the infusion bag containing TRODELVY solution can be stored refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours protected from light. After refrigeration, administer diluted solution at room temperature up to 25°C (77°F) within 8 hours (including infusion time).
Do Not Freeze or Shake.
Administration
- Administer TRODELVY as an intravenous infusion. Protect infusion bag from light. The infusion bag should be covered during administration to the patient until dosing is complete. It is not necessary to cover the infusion tubing or to use light-protective tubing during the infusion.
- An infusion pump may be used.
- Do not mix TRODELVY, or administer as an infusion, with other medicinal products.
- Upon completion of the infusion, flush the intravenous line with 20 mL 0.9% Sodium Chloride Injection, USP.

By using PrescriberAI, you agree to the AI Terms of Use.
Trodelvy prescribing information
WARNING: NEUTROPENIA AND DIARRHEA
- TRODELVY can cause severe, life-threatening, or fatal neutropenia. Withhold TRODELVY for absolute neutrophil count below 1500/mm 3 or neutropenic fever. Monitor blood cell counts periodically during treatment. Primary prophylaxis with G-CSF is recommended for all patients at increased risk of febrile neutropenia [see Dosage and Administration (2.3) ] . Initiate anti-infective treatment in patient with febrile neutropenia without delay [see Warnings and Precautions (5.1) ].
- TRODELVY can cause severe diarrhea. Monitor patients with diarrhea and give fluid and electrolytes as needed. At the onset of diarrhea, evaluate for infectious causes and, if negative, promptly initiate loperamide [see Warnings and Precautions (5.2) ]. If severe diarrhea occurs, withhold TRODELVY until resolved to ≤ Grade 1 and reduce subsequent doses [see Dosage and Administration (2.3) ].
| Boxed Warning | 03/2025 |
| Indications and Usage, Locally Advanced or Metastatic Urothelial Cancer – Accelerated Approval (text removed) (1.2) | 11/2024 |
| Dosage and Administration (2.2 , 2.3 , 2.4 ) | 03/2025 |
| Warnings and Precautions (5.1 ) | 03/2025 |
INDICATIONS AND USAGE
TRODELVY is a Trop-2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with:
Locally Advanced or Metastatic Breast Cancer
- Unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease. (1.1 , 14.1 )
- Unresectable locally advanced or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting. (1.1 , 14.2 )
Locally Advanced or Metastatic Breast Cancer
- TRODELVY is indicated for the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
- TRODELVY is indicated for the treatment of adult patients with unresectable locally advanced or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting.
DOSAGE AND ADMINISTRATION
- Do NOT substitute TRODELVY for or use with other drugs containing irinotecan or its active metabolite SN-38. (2.1 )
- For intravenous infusion only. Do not administer as an intravenous push or bolus.
- The recommended dose is 10 mg/kg once weekly on Days 1 and 8 of continuous 21-day treatment cycles until disease progression or unacceptable toxicity. (2.2 )
- Premedication for prevention of infusion reactions and prevention of chemotherapy-induced nausea and vomiting is recommended. (2.2 )
- Primary prophylaxis with G-CSF is recommended starting in the first cycle in all patients at increased risk of febrile neutropenia. (2.2 )
- Monitor patients during the infusion and for at least 30 minutes after completion of infusion. Treatment interruption and/or dose reduction may be needed to manage adverse reactions. (2.2 )
- See Full Prescribing Information for preparation and administration instructions. (2.4 )
Important Use Information
Do NOT substitute TRODELVY for or use with other drugs containing irinotecan or its active metabolite SN-38.
Recommended Dosage
The recommended dosage of TRODELVY is 10 mg/kg administered as an intravenous infusion once weekly on Days 1 and 8 of 21-day treatment cycles. Continue treatment until disease progression or unacceptable toxicity. Do not administer TRODELVY at doses greater than 10 mg/kg.
Administer TRODELVY as an intravenous infusion only. Do not administer as an intravenous push or bolus.
First infusion: Administer infusion over 3 hours. Observe patients during the infusion and for at least 30 minutes following the initial dose, for signs or symptoms of infusion-related reactions [see Warning and Precautions (5.3) ] .
Subsequent infusions: Administer infusion over 1 to 2 hours if prior infusions were tolerated. Observe patients during the infusion and for at least 30 minutes after infusion.
Premedication
Prior to each dose of TRODELVY, premedication for prevention of infusion reactions and prevention of chemotherapy-induced nausea and vomiting (CINV) is recommended.
- Premedicate with antipyretics, H1 and H2 blockers prior to infusion, and corticosteroids may be used for patients who had prior infusion reactions.
- Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK 1 receptor antagonist, as well as other drugs as indicated).
Prophylaxis for Neutropenia
Primary prophylaxis with granulocyte colony-stimulating factor (G-CSF) is recommended starting in the first cycle for all patients at increased risk of febrile neutropenia [see Warnings and Precautions (5.1) ] .
Dose Modifications for Adverse Reactions
Management of adverse reactions may require temporary interruption, dose reduction, or treatment discontinuation of TRODELVY as described in Tables 1 and 2. Do not re-escalate the TRODELVY dose after a dose reduction for adverse reactions has been made.
| Dose Level | Dosage and Schedule |
|---|---|
| Recommended starting dose | 10 mg/kg once weekly on Days 1 and 8 of 21-day treatment cycles |
| First dose reduction | Reduce to 7.5 mg/kg |
| Second dose reduction | Reduce to 5 mg/kg |
| Requirement for further dose reduction | Permanently discontinue TRODELVY |
The recommended dosage modifications for adverse reactions are provided in Table 2.
| Adverse reactions | Severity | Dose Modification |
|---|---|---|
| Neutropenia [see Warnings and Precautions (5.1) ] |
|
|
| Nausea/Vomiting/ Diarrhea [see Warnings and Precautions (5.2 , 5.4) ] |
|
|
| Infusion-Related Reaction [see Warnings and Precautions (5.3) ] |
|
|
|
| |
| Other Toxicities | Other Grade 3–4 toxicities of any duration despite optimal medical management |
|
Preparation and Administration
Reconstitution
- TRODELVY is a hazardous drug.
- Follow applicable special handling and disposal procedures 1 .
- Calculate the required dose (mg) of TRODELVY based on the patient's current body weight [see Dosage and Administration (2.2) ] .
- Using a sterile syringe, slowly inject 20 mL of 0.9% Sodium Chloride Injection, USP, into each 180 mg TRODELVY vial. Each vial contains overfill to compensate for liquid loss during preparation and after reconstitution, the total resulting volume delivers a concentration of 10 mg/mL .
- Gently swirl vials and allow to dissolve for up to 15 minutes. Do not shake. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be free of visible particulates, clear and yellow. Do not use the reconstituted solution if it is cloudy or discolored.
- Use immediately to prepare a diluted TRODELVY infusion solution.
Dilution
- Calculate the required amount of the reconstituted TRODELVY solution needed to obtain the appropriate dose according to the patient's body weight.
- Determine the final volume of the infusion solution to deliver the appropriate dose at a TRODELVY concentration range of 1.1 mg/mL to 3.4 mg/mL.
- Use 0.9% Sodium Chloride Injection, USP only since the stability of the reconstituted TRODELVY solution has not been determined with other infusion-based solutions. Use a polyvinyl chloride, polypropylene/polyethylene, polyolefin, or ethylene vinyl acetate infusion bag.
- Withdraw and discard the volume of 0.9% Sodium Chloride Injection, USP from the final infusion bag that is necessary to achieve the indicated TRODELVY concentration following the addition of the calculated amount of reconstituted TRODELVY solution.
- Withdraw the calculated amount of the reconstituted TRODELVY solution from the vial(s) using a syringe. Discard any unused portion remaining in the vial(s).
- To minimize foaming, slowly inject the calculated amount of reconstituted TRODELVY solution into the infusion bag. Do not shake the contents.
- If not used immediately, the infusion bag containing TRODELVY solution can be stored refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours protected from light. After refrigeration, administer diluted solution at room temperature up to 25°C (77°F) within 8 hours (including infusion time).
Do Not Freeze or Shake.
Administration
- Administer TRODELVY as an intravenous infusion. Protect infusion bag from light. The infusion bag should be covered during administration to the patient until dosing is complete. It is not necessary to cover the infusion tubing or to use light-protective tubing during the infusion.
- An infusion pump may be used.
- Do not mix TRODELVY, or administer as an infusion, with other medicinal products.
- Upon completion of the infusion, flush the intravenous line with 20 mL 0.9% Sodium Chloride Injection, USP.
DOSAGE FORMS AND STRENGTHS
For injection: 180 mg off-white to yellowish lyophilized powder in a single-dose vial.
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed. (8.2 )
Pregnancy
Risk Summary
Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. There are no available data in pregnant women to inform the drug-associated risk. TRODELVY contains a genotoxic component, SN-38, and is toxic to rapidly dividing cells [see Clinical Pharmacology (12.1) and Nonclinical Toxicology (13.1) ] . Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 – 4% and 15 – 20%, respectively.
Data
Animal data
There were no reproductive and developmental toxicology studies conducted with sacituzumab govitecan-hziy.
Lactation
Risk Summary
There is no information regarding the presence of sacituzumab govitecan-hziy or SN-38 in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment and for 1 month after the last dose of TRODELVY.
Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to the initiation of TRODELVY.
Contraception
Females
TRODELVY can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1) ] . Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose.
Males
Because of the potential for genotoxicity, advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRODELVY and for 3 months after the last dose.
Infertility
Females
Based on findings in animals, TRODELVY may impair fertility in females of reproductive potential [see Nonclinical Toxicology (13.1) ] .
Pediatric Use
Safety and effectiveness of TRODELVY have not been established in pediatric patients.
Geriatric Use
Of the 366 patients with TNBC who were treated with TRODELVY, 19% of patients were 65 years and 3% were 75 years and older. Patients 65 and older had an increased incidence of neutropenia, including fatal outcomes. No other differences in safety and effectiveness were observed between patients ≥ 65 years of age and younger patients.
Of the 322 patients with HR+/HER2- breast cancer who were treated with TRODELVY, 26% of patients were 65 years and older and 6% were 75 years and older. No overall differences in effectiveness were observed between patients ≥ 65 years of age and younger patients. There was a higher discontinuation rate due to adverse reactions in patients aged 65 years or older (14%) compared with younger patients (3%).
Hepatic Impairment
No adjustment to the starting dosage is required when administering TRODELVY to patients with mild hepatic impairment [see Clinical Pharmacology (12.3) ] .
The safety of TRODELVY in patients with moderate (total bilirubin > 1.5 to 3.0 × ULN) or severe (total bilirubin > 3.0 × upper limit of normal [ULN]) hepatic impairment has not been established. TRODELVY has not been tested in patients with AST or ALT > 3 ULN without liver metastases, or AST or ALT > 5 ULN with liver metastases. No recommendations can be made for the starting dosage in these patients.
CONTRAINDICATIONS
TRODELVY is contraindicated in patients who have experienced a severe hypersensitivity reaction to TRODELVY [see Warnings and Precautions (5.3) ] .
WARNINGS AND PRECAUTIONS
- Hypersensitivity and Infusion-Related Reactions: Hypersensitivity reactions including severe anaphylactic reactions have been observed. Monitor patients for infusion-related reactions. Permanently discontinue TRODELVY if severe or life-threatening reactions occur. (5.3 )
- Nausea/Vomiting: Use antiemetic preventive treatment and withhold TRODELVY for patients with Grade 3 nausea or Grade 3–4 vomiting at the time of scheduled treatment. (5.4 )
- Patients with Reduced UGT1A1 Activity: Individuals who are homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)•28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia following initiation of TRODELVY treatment. (5.5 )
- Embryo-Fetal Toxicity: TRODELVY can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception. (5.6 , 8.1 , 8.3 )
Neutropenia
TRODELVY can cause severe, life-threatening, or fatal neutropenia as early as the first cycle of treatment. Neutropenia occurred in 64% of patients treated with TRODELVY. Grade 3–4 neutropenia occurred in 49% of patients. Febrile neutropenia occurred in 6% of patients. The median time to first onset of neutropenia (including febrile neutropenia) was 16 days (range: 1 to 435 days). Neutropenia occurred earlier in patients with reduced UGT1A1 activity [see Warnings and Precautions (5.5) ] . Neutropenic colitis occurred in 1.4% of patients.
Primary prophylaxis with G-CSF is recommended starting in the first cycle of treatment in all patients at increased risk of febrile neutropenia, including older patients, patients with previous neutropenia, poor performance status, organ dysfunction, or multiple comorbidities.
Monitor absolute neutrophil count (ANC) during treatment. Withhold TRODELVY for ANC below 1500/mm 3 on Day 1 of any cycle or below 1000/mm 3 on Day 8 of any cycle. Withhold TRODELVY for neutropenic fever. Dose modifications may be required due to neutropenia. Treat neutropenia with G-CSF and administer prophylaxis in subsequent cycles as clinically indicated or indicated in Table 2 [see Dosage and Administration (2.3) ] .
Diarrhea
TRODELVY can cause severe diarrhea. Diarrhea occurred in 64% of all patients treated with TRODELVY. Grade 3–4 diarrhea occurred in 11% of all patients treated with TRODELVY. One patient had intestinal perforation following diarrhea. Diarrhea that led to dehydration and subsequent acute kidney injury occurred in 0.7% of all patients.
Withhold TRODELVY for Grade 3–4 diarrhea at the time of scheduled treatment administration and resume when resolved to ≤ Grade 1 [see Dosage and Administration (2.3) ].
At the onset of diarrhea, evaluate for infectious causes and if negative, promptly initiate loperamide, 4 mg initially followed by 2 mg with every episode of diarrhea for a maximum of 16 mg daily. Discontinue loperamide 12 hours after diarrhea resolves. Additional supportive measures (e.g., fluid and electrolyte substitution) may also be employed as clinically indicated.
Patients who exhibit an excessive cholinergic response to treatment with TRODELVY (e.g., abdominal cramping, diarrhea, salivation, etc.) can receive appropriate premedication (e.g., atropine) for subsequent treatments.
Hypersensitivity and Infusion-Related Reactions
TRODELVY can cause serious hypersensitivity reactions including life-threatening anaphylactic reactions. Severe signs and symptoms included cardiac arrest, hypotension, wheezing, angioedema, swelling, pneumonitis, and skin reactions [see Contraindications (4) ] .
Hypersensitivity reactions within 24 hours of dosing occurred in 35% of patients treated with TRODELVY. Grade 3–4 hypersensitivity occurred in 2% of patients treated with TRODELVY. The incidence of hypersensitivity reactions leading to permanent discontinuation of TRODELVY was 0.2%. The incidence of anaphylactic reactions was 0.2%.
Premedication for infusion reactions in patients receiving TRODELVY is recommended . Have medications and emergency equipment to treat infusion-related reactions, including anaphylaxis, available for immediate use when administering TRODELVY [see Dosage and Administration (2.2) ].
Closely monitor patients for hypersensitivity and infusion-related reactions during each TRODELVY infusion and for at least 30 minutes after completion of each infusion [see Dosage and Administration (2.3) ] .
Permanently discontinue TRODELVY for Grade 4 infusion-related reactions [see Dosage and Administration (2.3) ] .
Nausea and Vomiting
TRODELVY is emetogenic and can cause severe nausea and vomiting. Nausea occurred in 64% of all patients treated with TRODELVY. Grade 3–4 nausea occurred in 3% of patients.
Vomiting occurred in 35% of all patients treated with TRODELVY. Grade 3–4 vomiting occurred in 2% of these patients.
Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK 1 receptor antagonist as well as other drugs as indicated) for prevention of chemotherapy-induced nausea and vomiting (CINV) [ see Dosage and Administration (2.2) ].
Withhold TRODELVY doses for Grade 3 nausea or Grade 3–4 vomiting at the time of scheduled treatment administration and resume with additional supportive measures when resolved to ≤ Grade 1 [see Dosage and Administration (2.3) ].
Additional antiemetics and other supportive measures may also be employed as clinically indicated. All patients should be given take-home medications with clear instructions for prevention and treatment of nausea and vomiting.
Increased Risk of Adverse Reactions in Patients with Reduced UGT1A1 Activity
Patients homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)•28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia; and may be at increased risk for other adverse reactions when treated with TRODELVY.
The incidence of neutropenia and anemia was analyzed in 948 patients who received TRODELVY and had UGT1A1 genotype results. In patients homozygous for the UGT1A1 •28 allele (n=112), the incidence of Grade 3–4 neutropenia was 58%. In patients heterozygous for the UGT1A1•28 allele (n=420), the incidence of Grade 3–4 neutropenia was 49%. In patients homozygous for the wild-type allele (n=416), the incidence of Grade 3–4 neutropenia was 43% [see Clinical Pharmacology (12.5) ] . In patients homozygous for the UGT1A1 •28 allele, the incidence of Grade 3–4 anemia was 21%. In patients heterozygous for the UGT1A1•28 allele, the incidence of Grade 3–4 anemia was 10%. In patients homozygous for the wild-type allele, the incidence of Grade 3–4 anemia was 9%.
The median time to first neutropenia including febrile neutropenia was 9 days in patients homozygous for the UGT1A1•28 allele, 15 days in patients heterozygous for the UGT1A1•28 allele, and 20 days in patients homozygous for the wild-type allele. The median time to first anemia was 21 days in patients homozygous for the UGT1A1•28 allele, 25 days in patients heterozygous for the UGT1A1•28 allele, and 28 days in patients homozygous for the wild-type allele.
Closely monitor patients with known reduced UGT1A1 activity for adverse reactions. Withhold or permanently discontinue TRODELVY based on clinical assessment of the onset, duration and severity of the observed adverse reactions in patients with evidence of acute early-onset or unusually severe adverse reactions, which may indicate reduced UGT1A1 enzyme activity [see Dosage and Administration (2.3) ].
Embryo-Fetal Toxicity
Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. TRODELVY contains a genotoxic component, SN-38, and targets rapidly dividing cells [see Clinical Pharmacology (12.1) and Nonclinical Toxicology (13.1) ]. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRODELVY and for 3 months after the last dose [see Use in Specific Populations (8.1 , 8.3) ] .
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Neutropenia [see Warnings and Precautions (5.1) ]
- Diarrhea [see Warnings and Precautions (5.2) ]
- Hypersensitivity and Infusion-Related Reactions [see Warnings and Precautions (5.3) ]
- Nausea and Vomiting [see Warnings and Precautions (5.4) ]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The pooled safety population described in the Warnings and Precautions section reflect exposure to TRODELVY in 1063 patients, which included 366 patients with mTNBC and 322 patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) breast cancer from IMMU-132-01, ASCENT, and TROPiCS-02; and 375 patients with other tumor types. TRODELVY was administered as an intravenous infusion once weekly on Days 1 and 8 of 21-day treatment cycles at doses of 10 mg/kg until disease progression or unacceptable toxicity. Among the 1063 patients treated with TRODELVY, the median duration of treatment was 4.1 months (range: 0 to 63 months). In this pooled safety population, the most common (≥ 25%) adverse reactions including laboratory abnormalities were decreased leukocyte count (84%), decreased neutrophil count (75%), decreased hemoglobin (69%), diarrhea (64%), nausea (64%), decreased lymphocyte count (63%), fatigue (51%), alopecia (45%), constipation (37%), increased glucose (37%), decreased albumin (35%), vomiting (35%), decreased appetite (30%), decreased creatinine clearance (28%), increased alkaline phosphatase (28%), decreased magnesium (27%), decreased potassium (26%), and decreased sodium (26%).
Locally Advanced or Metastatic Triple-Negative Breast Cancer
ASCENT Study
The safety of TRODELVY was evaluated in a randomized, active-controlled, open-label study (ASCENT) in patients with mTNBC who had previously received a taxane and at least two prior chemotherapies. Patients were randomized (1:1) to receive either TRODELVY (n=258) or single agent chemotherapy (n=224) and were treated until disease progression or unacceptable toxicity [see Clinical Studies (14.1) ] . For patients treated with TRODELVY, the median duration of treatment was 4.4 months (range: 0 to 23 months).
Serious adverse reactions occurred in 27% of patients receiving TRODELVY. Serious adverse reactions in > 1% of patients receiving TRODELVY included neutropenia (7%), diarrhea (4%), and pneumonia (3%). Fatal adverse reactions occurred in 1.2% of patients who received TRODELVY, including respiratory failure (0.8%) and pneumonia (0.4%). TRODELVY was permanently discontinued for adverse reactions in 5% of patients. Adverse reactions leading to permanent discontinuation in ≥ 1 % of patients who received TRODELVY were pneumonia (1%) and fatigue (1%).
Adverse reactions leading to a treatment interruption of TRODELVY occurred in 63% of patients. The most frequent (≥5%) adverse reactions leading to a treatment interruption were neutropenia (47%), diarrhea (5%), respiratory infection (5%), and leukopenia (5%).
Adverse reactions leading to a dose reduction of TRODELVY occurred in 22% of patients. The most frequent (>4%) adverse reactions leading to a dose reduction were neutropenia (11%) and diarrhea (5%).
Granulocyte-colony stimulating factor (G-CSF) was used in 44% of patients who received TRODELVY.
Tables 3 and 4 summarize adverse reactions and laboratory abnormalities, respectively, in the ASCENT study.
| TRODELVY (n=258) | Single Agent Chemotherapy (n=224) | |||
|---|---|---|---|---|
| Adverse Reaction | All Grades % | Grade 3 – 4 % | All Grades % | Grade 3 – 4 % |
| •Single agent chemotherapy included one of the following single-agents: eribulin (n=139), capecitabine (n=33), gemcitabine (n=38), or vinorelbine (except if patient had ≥ Grade 2 neuropathy, n=52). | ||||
| Graded per NCI CTCAE v.5.0. | ||||
| Gastrointestinal disorders | ||||
| Diarrhea | 59 | 11 | 17 | 1 |
| Nausea | 57 | 3 | 26 | 0.4 |
| Vomiting | 33 | 2 | 16 | 1 |
| Constipation | 37 | 0.4 | 23 | 0 |
| Abdominal Pain | 30 | 3 | 12 | 1 |
| Stomatitis Including stomatitis, glossitis, mouth ulceration, and mucosal inflammation | 17 | 2 | 13 | 1 |
| General disorders and administration site conditions | ||||
| Fatigue Including fatigue and asthenia | 65 | 6 | 50 | 9 |
| Pyrexia | 15 | 0.4 | 14 | 2 |
| Infections and infestation | ||||
| Urinary tract infection | 13 | 0.4 | 8 | 0.4 |
| Upper respiratory tract infection | 12 | 0 | 3 | 0 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 28 | 2 | 21 | 1 |
| Musculoskeletal and connective tissue disorders | ||||
| Back pain | 16 | 1 | 14 | 2 |
| Arthralgia | 12 | 0.4 | 7 | 0 |
| Nervous system disorders | ||||
| Headache | 18 | 0.8 | 13 | 0.4 |
| Dizziness | 10 | 0 | 7 | 0 |
| Psychiatric disorders | ||||
| Insomnia | 11 | 0 | 5 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||||
| Cough | 24 | 0 | 18 | 0.4 |
| Skin and subcutaneous tissue disorders | ||||
| Alopecia | 47 | 0 | 16 | 0 |
| Rash | 12 | 0.4 | 5 | 0.4 |
| Pruritus | 10 | 0 | 3 | 0 |
| Laboratory Abnormality | TRODELVY (n=258) | Single Agent Chemotherapy (n=224) | ||
|---|---|---|---|---|
| All Grades (%) | Grade 3 – 4 (%) | All Grades (%) | Grade 3 – 4 (%) | |
| Hematology | ||||
| Decreased hemoglobin | 94 | 9 | 57 | 6 |
| Decreased lymphocyte count | 88 | 31 | 40 | 24 |
| Decreased leukocyte count | 86 | 41 | 53 | 25 |
| Decreased neutrophil count | 78 | 49 | 48 | 36 |
| Decreased platelet count | 23 | 1.2 | 25 | 2.7 |
| Chemistry | ||||
| Increased glucose | 49 | 2.3 | 43 | 2.8 |
| Decreased calcium | 36 | 1.6 | 21 | 1.4 |
| Decreased magnesium | 33 | 0.4 | 20 | 0 |
| Decreased potassium | 33 | 4.3 | 28 | 0.9 |
| Increased albumin | 32 | 0.8 | 25 | 1.4 |
| Increased aspartate aminotransferase | 27 | 1.2 | 32 | 1.4 |
| Increased alanine aminotransferase | 26 | 1.2 | 26 | 1.8 |
| Increased alkaline phosphatase | 26 | 0 | 17 | 0.5 |
| Decreased phosphate | 26 | 7.8 | 20 | 3.3 |
| Decreased sodium | 22 | 0.4 | 17 | 0.5 |
| Increased lactate dehydrogenase | 18 | 0 | 22 | 0 |
| Decreased glucose | 10 | 0 | 3.2 | 0 |
Study IMMU-132-01
The safety of TRODELVY was evaluated in a single-arm, open-label study (IMMU-132-01) in patients with mTNBC and other malignancies, which included 108 patients with mTNBC who had received at least two prior anticancer therapies for metastatic disease [see Clinical Studies (14.1) ] . TRODELVY was administered as an intravenous infusion once weekly on Days 1 and 8 of 21-day treatment cycles at doses up to 10 mg/kg until disease progression or unacceptable toxicity. The median treatment duration in these 108 patients was 5.1 months (range: 0 to 51 months).
Serious adverse reactions occurred in 31% of the patients. Serious adverse reactions in > 1% of patients receiving TRODELVY included febrile neutropenia (6%) vomiting (5%), nausea (3%), dyspnea (3%), diarrhea (4%), anemia (2%), pleural effusion, neutropenia, pneumonia, dehydration (each 2%).
TRODELVY was permanently discontinued for adverse reactions in 2% of patients. Adverse reactions leading to permanent discontinuation were anaphylaxis, anorexia/fatigue, headache (each 0.9%). Forty- five percent (45%) of patients experienced an adverse reaction leading to treatment interruption. The most common adverse reaction leading to treatment interruption was neutropenia (33%). Adverse reactions leading to dose reduction occurred in 33% of patients treated with TRODELVY, with 24% having one dose reduction, and 9% with two dose reductions. The most common adverse reaction leading to dose reductions was neutropenia/febrile neutropenia.
Tables 5 and 6 summarize adverse reactions and laboratory abnormalities occurring in ≥ 10% of patients with mTNBC in the IMMU-132-01 study.
| Adverse Reaction | TRODELVY (n=108) | |
|---|---|---|
| Grade 1–4 (%) | Grade 3–4 (%) | |
| Graded per NCI CTCAE v. 4.0 | ||
| Any adverse reaction | 100 | 71 |
| Gastrointestinal disorders | 95 | 21 |
| Nausea | 69 | 6 |
| Diarrhea | 63 | 9 |
| Vomiting | 49 | 6 |
| Constipation | 34 | 1 |
| Abdominal pain Including abdominal pain, distention, pain (upper), discomfort, tenderness. | 26 | 1 |
| Mucositis Including stomatitis, esophagitis, and mucosal inflammation | 14 | 1 |
| General disorders and administration site conditions | 77 | 9 |
| Fatigue Including fatigue and asthenia. | 57 | 8 |
| Edema Including edema; and peripheral, localized, and periorbital edema | 19 | 0 |
| Pyrexia | 14 | 0 |
| Metabolism and nutrition disorders | 68 | 22 |
| Decreased appetite | 30 | 1 |
| Dehydration | 13 | 5 |
| Skin and subcutaneous tissue disorders | 63 | 4 |
| Alopecia | 38 | 0 |
| Rash Including rash; maculopapular, erythematous, generalized rash; dermatitis acneiform; skin disorder, irritation, and exfoliation | 31 | 3 |
| Pruritus | 17 | 0 |
| Dry Skin | 15 | 0 |
| Nervous system disorders | 56 | 4 |
| Headache | 23 | 1 |
| Dizziness | 22 | 0 |
| Neuropathy Including gait disturbance, hypoesthesia, muscular weakness, paresthesia, peripheral and sensory neuropathy | 24 | 0 |
| Dysgeusia | 11 | 0 |
| Infections and infestations | 55 | 12 |
| Urinary Tract Infection | 21 | 3 |
| Respiratory Infection Including lower and upper respiratory tract infection, pneumonia, influenza, viral upper respiratory infection, bronchitis and respiratory syncytial virus infection | 26 | 3 |
| Musculoskeletal and connective tissue disorders | 54 | 1 |
| Back pain | 23 | 0 |
| Arthralgia | 17 | 0 |
| Pain in extremity | 11 | 0 |
| Respiratory, thoracic and mediastinal disorders | 54 | 5 |
| Cough Includes cough and productive cough | 22 | 0 |
| Dyspnea Includes dyspnea and exertional dyspnea | 21 | 3 |
| Psychiatric disorders | 26 | 1 |
| Insomnia | 13 | 0 |
| Laboratory Abnormality | TRODELVY (n=108) | |
|---|---|---|
| All Grades (%) | Grade 3–4 (%) | |
| Hematology | ||
| Decreased hemoglobin | 93 | 6 |
| Decreased leukocyte count | 91 | 26 |
| Decreased neutrophil count | 82 | 32 |
| Increased activated partial thromboplastin time | 60 | 12 |
| Decreased platelet count | 30 | 3 |
| Chemistry | ||
| Increased alkaline phosphatase | 57 | 2 |
| Decreased magnesium | 51 | 3 |
| Decreased calcium | 49 | 3 |
| Increased aspartate aminotransferase | 45 | 3 |
| Decreased albumin | 39 | 1 |
| Increased alanine aminotransferase | 35 | 2 |
| Increased glucose | 31 | 2.8 |
| Decreased phosphate | 29 | 5 |
| Decrease magnesium | 27 | 1.9 |
| Decreased phosphate | 27 | 6.5 |
| Decreased sodium | 25 | 4.7 |
| Decreased potassium | 24 | 3.7 |
| Decreased glucose | 19 | 2 |
Locally Advanced or Metastatic HR-Positive, HER2-Negative Breast Cancer
TROPiCS-02 Study
The safety of TRODELVY was evaluated in a randomized, active-controlled, open-label, study (TROPiCS-02) in patients with unresectable locally advanced or metastatic HR-positive, HER2-negative breast cancer whose disease has progressed after the following in any setting: a CDK 4/6 inhibitor, endocrine therapy, and a taxane; patients received at least two prior chemotherapies in the metastatic setting (one of which could be in the neoadjuvant or adjuvant setting if progression occurred within 12 months). Patients were randomized (1:1) to receive either TRODELVY (n=268) or single agent chemotherapy (n=249) and were treated until disease progression or unacceptable toxicity [see Clinical Studies (14.2) ] . For patients treated with TRODELVY, the median duration of treatment was 4.1 months (range: 0 to 63 months).
Serious adverse reactions occurred in 28% of patients receiving TRODELVY. Serious adverse reactions in > 1% of patients receiving TRODELVY included diarrhea (5%), febrile neutropenia (4%), neutropenia (3%), abdominal pain, colitis, neutropenic colitis, pneumonia, and vomiting (each 2%). Fatal adverse reactions occurred in 2% of patients who received TRODELVY including arrhythmia, COVID-19, nervous system disorder, pulmonary embolism, and septic shock (each 0.4%). TRODELVY was permanently discontinued for adverse reactions in 6% of patients. The most frequent (≥ 0.5%) adverse reactions leading to permanent discontinuation in patients who received TRODELVY were asthenia, general physical health deterioration, and neutropenia (each 0.7%).
Adverse reactions leading to a treatment interruptions of TRODELVY occurred in 66% of patients. The most frequent (≥ 5%) adverse reaction leading to treatment interruption was neutropenia (50%).
Adverse reaction leading to a dose reduction of TRODELVY occurred in 33% of patients. The most frequent (> 5%) adverse reaction leading to dose reduction were neutropenia (16%) and diarrhea (8%). G-CSF was used in 54% of patients who received TRODELVY.
Tables 7 and 8 summarize adverse reactions and laboratory abnormalities in the TROPiCS-02 study.
| TRODELVY (n=268) | Single Agent Chemotherapy (n=249) | |||
|---|---|---|---|---|
| Adverse Reaction | All Grades % | Grade 3 – 4 % | All Grades % | Grade 3 – 4 % |
| •Single agent chemotherapy included one of the following single-agents: eribulin (n=130), vinorelbine (n=63), gemcitabine (n=56), or capecitabine (n=22). | ||||
| Graded per NCI CTCAE v.5.0. | ||||
| Gastrointestinal disorders | ||||
| Diarrhea | 62 | 10 | 23 | 1 |
| Nausea | 59 | 1 | 35 | 3 |
| Constipation | 34 | 1 | 25 | 0 |
| Vomiting | 23 | 1 | 16 | 2 |
| Abdominal Pain | 20 | 0 | 14 | 0 |
| Dyspepsia Including dyspepsia, gastroesophageal reflux disease. | 11 | 0 | 6 | 0 |
| General disorders and administration site conditions | ||||
| Fatigue Including fatigue, asthenia. | 60 | 8 | 51 | 4 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 21 | 2 | 21 | 0 |
| Hypokalemia | 10 | 2 | 4 | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 15 | 0 | 12 | 0 |
| Nervous system disorders | ||||
| Headache | 16 | 1 | 15 | 1 |
| Respiratory, thoracic and mediastinal disorders | ||||
| Dyspnea Including dyspnea; exertional dyspnea | 20 | 0 | 17 | 0 |
| Cough | 12 | 0 | 7 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Alopecia | 48 | 0 | 19 | 0 |
| Pruritus | 12 | 0 | 2 | 0 |
Other clinically significant adverse reactions in TROPiCS-02 (≤ 10%) include: hypotension (5%), pain (5%), rhinorrhea (5%), hypocalcemia (3%), nasal congestion (3%), skin hyperpigmentation, (3%), colitis or neutropenic colitis (2%), hyponatremia (2%), pneumonia (2%), proteinuria (1%), enteritis (0.4%).
| Laboratory Abnormality | TRODELVY (n=268) | Single Agent Chemotherapy (n=249) | ||
|---|---|---|---|---|
| All Grades (%) | Grade 3 – 4 (%) | All Grades (%) | Grade 3 – 4 (%) | |
| Hematology | ||||
| Decreased leukocyte count | 88 | 38 | 73 | 26 |
| Decreased neutrophil count | 83 | 53 | 67 | 40 |
| Decreased hemoglobin | 73 | 8 | 59 | 5 |
| Decreased lymphocyte count | 65 | 21 | 47 | 14 |
| Decreased platelet count | 21 | 1 | 30 | 4 |
| Eosinophilia | 13 | 0 | 4 | 0 |
| Chemistry | ||||
| Increased glucose | 37 | 0 | 31 | 0 |
| Decreased albumin | 32 | 0 | 27 | 0.4 |
| Decreased creatinine clearance | 24 | 2 | 19 | 1 |
| Increased alkaline phosphatase | 23 | 0 | 23 | 1 |
| Decreased potassium | 22 | 3 | 12 | 0.4 |
| Increased alanine aminotransferase | 21 | 1 | 31 | 2 |
| Decreased sodium | 19 | 1 | 17 | 0.4 |
| Decreased magnesium | 18 | 0 | 15 | 0 |
| Decreased phosphate | 17 | 0 | 10 | 0 |
| Increased phosphate | 16 | 0 | 16 | 0 |
| Increased lactate dehydrogenase | 16 | 0 | 28 | 0 |
| Increased aspartate aminotransferase | 15 | 2 | 25 | 1 |
| Increased potassium | 14 | 2 | 9 | 0 |
DRUG INTERACTIONS
- UGT1A1 inhibitors or inducers: Avoid concomitant use. (7 )
Effect of Other Drugs on TRODELVY
UGT1A1 Inhibitors
Concomitant administration of TRODELVY with inhibitors of UGT1A1 may increase the incidence of adverse reactions due to potential increase in systemic exposure to SN-38 [see Warning and Precaution (5.5) and Clinical Pharmacology (12.3 , 12.5) ] . Avoid administering UGT1A1 inhibitors with TRODELVY.
UGT1A1 Inducers
Exposure to SN-38 may be reduced in patients concomitantly receiving UGT1A1 enzyme inducers [see Warning and Precaution (5.5) and Clinical Pharmacology (12.3 , 12.5) ] . Avoid administering UGT1A1 inducers with TRODELVY.
DESCRIPTION
Sacituzumab govitecan-hziy is a Trop-2 directed antibody and topoisomerase inhibitor conjugate, composed of the following three components:
- the humanized monoclonal antibody, hRS7 IgG1κ (also called sacituzumab), which binds to Trop-2 (the trophoblast cell-surface antigen-2);
- the drug SN-38, a topoisomerase inhibitor;
- a hydrolysable linker (called CL2A), which links the humanized monoclonal antibody to SN-38.
The recombinant monoclonal antibody is produced by mammalian (murine myeloma) cells, while the small molecule components SN-38 and CL2A are produced by chemical synthesis. Sacituzumab govitecan-hziy contains on average 7 to 8 molecules of SN-38 per antibody molecule. Sacituzumab govitecan-hziy has a molecular weight of approximately 160 kilodaltons. Sacituzumab govitecan-hziy has the following chemical structure.
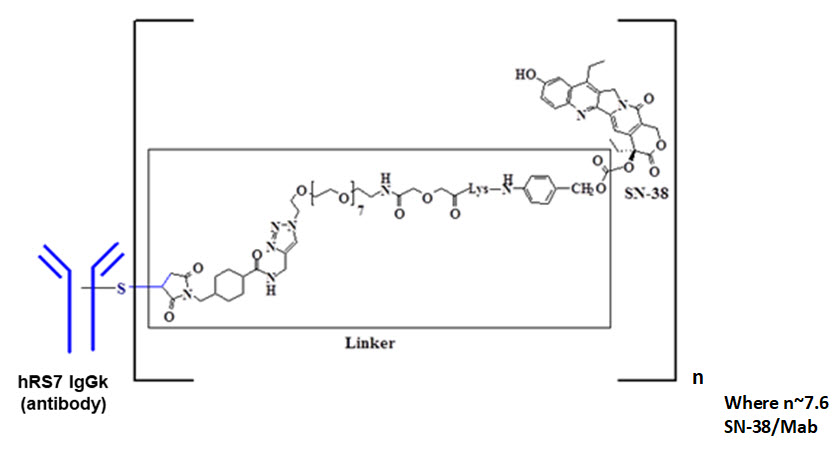 |
TRODELVY (sacituzumab govitecan-hziy) for injection is a sterile, preservative-free, off-white to yellowish lyophilized powder for intravenous use in a 50 mL clear glass single-dose vial, with a rubber stopper and crimp-sealed with an aluminum flip-off cap.
Each single-dose vial of TRODELVY delivers 180 mg sacituzumab govitecan-hziy, 71.7 mg 2-(N-morpholino) ethane sulfonic acid (MES), 1.8 mg polysorbate 80 and 153.99 mg trehalose. Reconstitution with 20 mL of 0.9% Sodium Chloride Injection, USP, results in a concentration of 10 mg/mL with a pH of 6.5.
CLINICAL PHARMACOLOGY
Mechanism of Action
Sacituzumab govitecan-hziy is a Trop-2-directed antibody-drug conjugate. Sacituzumab is a humanized antibody that recognizes Trop-2. The small molecule, SN-38, is a topoisomerase I inhibitor, which is covalently attached to the antibody by a linker. Pharmacology data suggest that sacituzumab govitecan-hziy binds to Trop-2-expressing cancer cells and is internalized with the subsequent release of SN-38 via hydrolysis of the linker. SN-38 interacts with topoisomerase I and prevents re-ligation of topoisomerase I-induced single strand breaks. The resulting DNA damage leads to apoptosis and cell death. Sacituzumab govitecan-hziy decreased tumor growth in mouse xenograft models of triple-negative breast cancer.
Pharmacodynamics
The TRODELVY exposure-response relationships and pharmacodynamic time course for efficacy have not been fully characterized.
Cardiac electrophysiology
The maximum mean change from baseline was 9.7 msec (the upper bound of the two-sided 90% confidence interval is 16.8 msec) at the recommended dose. A positive exposure-response relationship was observed between QTc increases and SN-38 concentrations.
Pharmacokinetics
The serum pharmacokinetics of sacituzumab govitecan-hziy and SN-38 were evaluated in patients with mBC who received sacituzumab govitecan-hziy as a single agent at a dose of 10 mg/kg. The pharmacokinetic parameters of sacituzumab govitecan-hziy and free SN-38 are presented in Table 9.
| Sacituzumab govitecan-hziy (N=693) | Free SN-38 (N=681) | |
|---|---|---|
| C max : maximum serum concentration from 0–168 hours after the first dose | ||
| AUC 0–168 : area under serum concentration curve through 168 hours after the first dose | ||
| C max [ng/mL] | 239000 (11%) | 98.0 (45%) |
| AUC 0–168 [ng•h/mL] | 5640000 (22%) | 3696 (56%) |
Distribution
Based on population pharmacokinetic analysis, steady state volume of distribution of sacituzumab govetican-hziy is 3.6L.
Elimination
The median elimination half-life (t 1/2 ) of sacituzumab govitecan-hziy and free SN-38 in patients with metastatic triple negative breast cancer was 23.4 and 17.6 hours, respectively. Based on population pharmacokinetic analysis, the estimated mean (%CV) clearance of the sacituzumab govitecan-hziy is 0.13 L/h (12%).
Metabolism
No metabolism studies with sacituzumab govitecan-hziy have been conducted. SN-38 (the small molecule moiety of sacituzumab govitecan-hziy) is metabolized via UGT1A1. The glucuronide metabolite of SN-38 (SN-38G) was detectable in the serum of patients.
Specific Populations
Pharmacokinetic analyses in patients treated with TRODELVY did not identify an effect of age (27 to 88 years), race (White, Black, or Asian), or mild renal impairment to moderate renal impairment (CLcr 30 to 89 mL/min) on the pharmacokinetics of sacituzumab govitecan-hziy. Renal elimination is known to contribute minimally to the excretion of SN-38, the small molecule moiety of sacituzumab govitecan-hziy. There are no data on the pharmacokinetics of sacituzumab govitecan-hziy in patients with severe renal impairment (CLcr 15 to 29 mL/min), or end-stage renal disease (CLcr < 15 mL/min).
Patients with Hepatic Impairment
The exposure of sacituzumab govitecan-hziy is similar in patients with mild hepatic impairment (total bilirubin ≤ ULN with AST > ULN, or bilirubin >1.0 to ≤ 1.5 ULN with any AST; n=257) to patients with normal hepatic function (total bilirubin and AST < ULN; n=526).
Sacituzumab govitecan-hziy and free SN-38 exposures are unknown in patients with moderate (total bilirubin > 1.5 to 3.0 × ULN) or severe (total bilirubin > 3.0 × ULN) hepatic impairment.
Drug Interaction Studies
No drug-drug interaction studies were conducted with sacituzumab govitecan-hziy or its components. Inhibitors or inducers of UGT1A1 may increase or decrease SN-38 exposure, respectively [see Drug Interactions (7) ] .
Pharmacogenomics
SN-38 is metabolized via UGT1A1 [ see Clinical Pharmacology (12.3) ]. Genetic variants of the UGT1A1 gene such as the UGT1A1•28 allele lead to reduced UGT1A1 enzyme activity. Individuals who are homozygous or heterozygous for the UGT1A1•28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia from TRODELVY compared to individuals who are wildtype (•1/•1) [see Warnings and Precautions (5.5) ]. Approximately 20% of the Black or African American population, 10% of the White population, and 2% of the East Asian population are homozygous for the UGT1A1•28 allele (•28/•28). Approximately 40% of the Black or African American population, 50% of the White population, and 25% of the East Asian population are heterozygous for the UGT1A1•28 allele (•1/•28). Decreased function alleles other than UGT1A1•28 may be present in certain populations.
Immunogenicity
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of TRODELVY.
During the median 4-month treatment period across clinical studies in patients treated with TRODELVY, 9 (1.1%) of 785 patients developed antibodies to sacituzumab govitecan; 6 of these patients (0.8% of all patients treated with TRODELVY) had neutralizing antibodies against sacituzumab govitecan. Because of the low occurrence of anti-drug antibodies, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of sacituzumab govitecan is unknown.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with sacituzumab govitecan-hziy.
SN-38 was clastogenic in an in vitro mammalian cell micronucleus test in Chinese hamster ovary cells and was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay.
Fertility studies with sacituzumab govitecan-hziy have not been conducted. In a repeat-dose toxicity study in cynomolgus monkeys, intravenous administration of sacituzumab govitecan-hziy on Day 1 and Day 4 resulted in endometrial atrophy, uterine hemorrhage, increased follicular atresia of the ovary, and atrophy of vaginal epithelial cells at doses ≥ 60 mg/kg (≥ 6 times the human recommended dose of 10 mg/kg based on body weight).
CLINICAL STUDIES
Locally Advanced or Metastatic Triple-Negative Breast Cancer
ASCENT
Efficacy was evaluated in a multicenter, open-label, randomized study (ASCENT; NCT02574455) conducted in 529 patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who had relapsed after at least two prior chemotherapies for breast cancer (one of which could be in the neoadjuvant or adjuvant setting provided progression occurred within a 12 month period). All patients received previous taxane treatment in either the adjuvant, neoadjuvant, or advanced stage unless there was a contraindication or intolerance to taxanes during or at the end of the first taxane cycle. Magnetic resonance imaging (MRI) to determine brain metastases was required prior to enrollment for patients with known or suspected brain metastases. Patients with brain metastases were allowed to enroll up to a pre-defined maximum of 15% of patients in the ASCENT study. Patients with known Gilbert's disease or bone-only disease were excluded.
Patients were randomized (1:1) to receive TRODELVY 10 mg/kg as an intravenous infusion on Days 1 and 8 of a 21-day (n=267) or physician's choice of single agent chemotherapy (n=262). Single agent chemotherapy was determined by the investigator before randomization from one of the following choices: eribulin (n=139), capecitabine (n=33), gemcitabine (n=38), or vinorelbine (n=52).
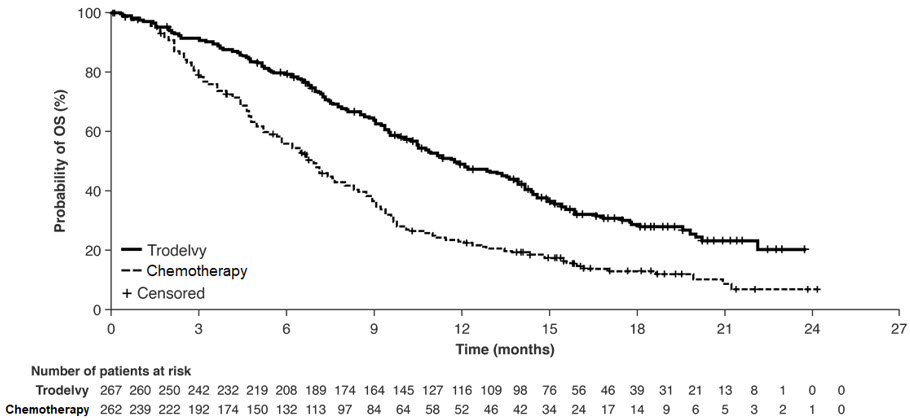
Patients were treated until disease progression or unacceptable toxicity. The major efficacy outcome was progression-free survival (PFS) in patients without brain metastases at baseline (i.e., BMNeg) as measured by a blinded, independent, centralized review assessed using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 criteria. Additional efficacy measures included PFS for the full population (all patients with and without brain metastases) and overall survival (OS).
The median age of patients in the full population (n = 529) was 54 years (range: 27 to 82 years); 99.6% were female; 79% were White, 12% were Black/African American; and 81% of patients were < 65 years of age. All patients had an ECOG performance status of 0 (43%) or 1 (57%). Forty-two percent of patients had hepatic metastases, 9% were BRCA1/BRCA2 mutational status positive, and 70% were TNBC at diagnosis. Twelve percent had baseline brain metastases previously treated and stable (n=61; 32 on TRODELVY arm and 29 on single agent chemotherapy arm). Overall, 29% of patients had received prior PD-1/PD-L1 therapy. Thirteen percent of patients in the TRODELVY group in the full population received only 1 prior line of systemic therapy in the metastatic setting.
The efficacy results are summarized in Table 10 and are shown in Figure 1 and Figure 2. Efficacy results for the subgroup of patients who had received only 1 prior line of systemic therapy in the metastatic setting (in addition to having disease recurrence or progression within 12 months of neoadjuvant/adjuvant systemic therapy) were consistent with those who had received at least two prior lines in the metastatic setting.
| All Randomized Patients | ||
|---|---|---|
| TRODELVY n=267 | Single Agent Chemotherapy n=262 | |
| CI = Confidence Interval | ||
| Progression-Free Survival PFS is defined as the time from the date of randomization to the date of the first radiological disease progression or death due to any cause, whichever comes first. per BICR | ||
| Disease Progression or Death (%) | 190 (71%) | 171 (65%) |
| Median PFS in months (95% CI) | 4.8 (4.1, 5.8) | 1.7 (1.5, 2.5) |
| Hazard ratio Stratified log-rank test adjusted for stratification factors: number of prior chemotherapies, presence of known brain metastases at study entry, and region. (95% CI) | 0.43 (0.35, 0.54) | |
| p-value | <0.0001 | |
| Overall Survival | ||
| Deaths (%) | 179 (67%) | 206 (79%) |
| Median OS in months (95% CI) | 11.8 (10.5, 13.8) | 6.9 (5.9, 7.6) |
| Hazard ratio(95% CI) | 0.51 (0.41, 0.62) | |
| p-value | <0.0001 | |
Figure 1: Kaplan-Meier Plot of PFS by BICR (All Randomized Patients) in ASCENT
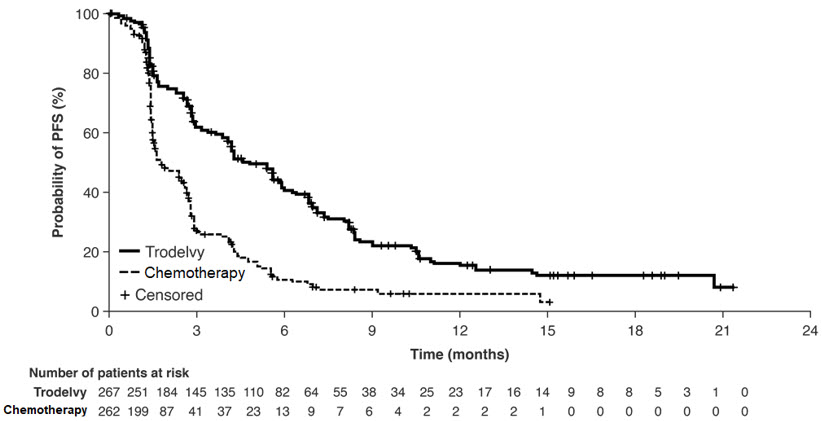
Figure 2: Kaplan-Meier Plot of OS (All Randomized Patients) in ASCENT
An exploratory analysis of PFS in patients with previously treated, stable brain metastases showed a stratified HR of 0.65 (95% CI: 0.35, 1.22). The median PFS in the TRODELVY arm was 2.8 months (95% CI: 1.5, 3.9) and the median PFS with single agent chemotherapy was 1.6 months (95% CI: 1.3, 2.9). Exploratory OS analysis in the same population showed a stratified HR of 0.87 (95% CI: 0.47, 1.63). The median OS in the TRODELVY arm was 6.8 months (95% CI: 4.7, 14.1) and the median OS with single agent chemotherapy was 7.4 months (95% CI: 4.7, 11.1).
IMMU-132-01
The efficacy of TRODELVY was evaluated in a multicenter, single-arm, study (NCT01631552) that enrolled 108 patients with metastatic triple-negative breast cancer (mTNBC) who had received at least two prior anticancer therapies for metastatic disease. Patients with bulky disease, defined as a mass > 7 cm, were not eligible. Patients with treated brain metastases not receiving high dose steroids (> 20 mg prednisone or equivalent) for at least four weeks were eligible. Patients with known Gilbert's disease were excluded.
Patients received TRODELVY 10 mg/kg intravenously on Days 1 and 8 of a 21-day treatment cycle. Patients were treated with TRODELVY until disease progression or intolerance to the therapy. Tumor imaging was obtained every 8 weeks, with confirmatory CT/MRI scans obtained 4–6 weeks after an initial partial or complete response, until progression requiring treatment discontinuation. Major efficacy outcome measures were investigator assessed overall response rate (ORR) using RECIST 1.1 and duration of response.
The median age was 55 years (range: 31 to 80 years); 87% of patients were younger than 65 years. The majority of patients were female (99%) and White (76%). At study entry, all patients had an ECOG performance status of 0 (29%) or 1 (71%). Seventy-six percent had visceral disease, 42% had hepatic metastases, 56% had lung/pleura metastases, and 2% had brain metastases. Twelve patients (11%) had Stage IV disease at the time of initial diagnosis.
The median number of prior systemic therapies received in the metastatic setting was 3 (range: 2 to 10). Prior chemotherapies in the metastatic setting included carboplatin or cisplatin (69%), gemcitabine (55%), paclitaxel or docetaxel (53%), capecitabine (51%), eribulin (45%), doxorubicin (24%), vinorelbine (16%), cyclophosphamide (19%), and ixabepilone (8%).
Overall, 98% of patients had received prior taxanes and 86% had received prior anthracyclines either in the (neo)adjuvant or metastatic setting.
Table 11 summarizes the efficacy results.
| TRODELVY (N=108) | |
|---|---|
| CI: confidence interval | |
| +: denotes ongoing | |
| Overall Response Rate investigator assessment | |
| ORR (95% CI) | 33.3% (24.6, 43.1) |
| Complete response | 2.8% |
| Partial response | 30.6% |
| Response duration | |
| Number of responders | 36 |
| Median, Months (95% CI) | 7.7 (4.9, 10.8) |
| Range, Months | 1.9+, 30.4+ |
| % with duration ≥ 6 months | 55.6% |
| % with duration ≥ 12 months | 16.7% |
Locally Advanced or Metastatic HR-Positive, HER2-Negative Breast Cancer
TROPiCS-02 Study
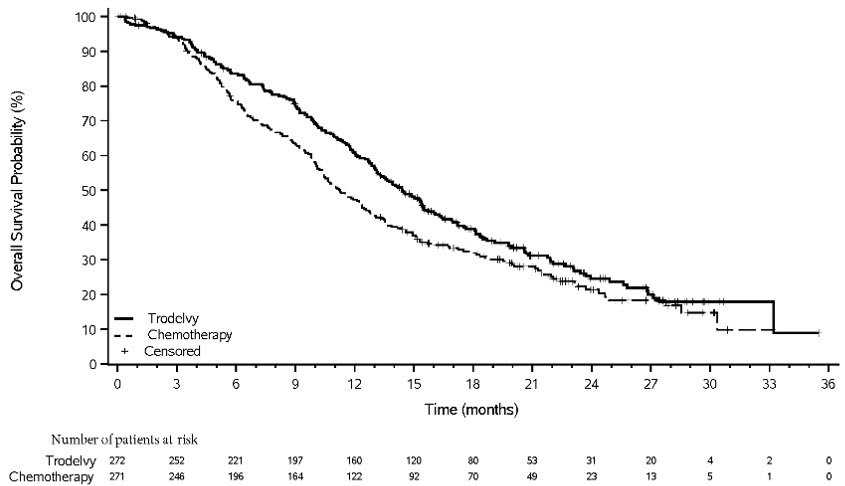
The efficacy of TRODELVY was evaluated in a multicenter, open label, randomized study (TROPiCS-02; NCT03901339) conducted in 543 patients with unresectable locally advanced or metastatic HR-positive, HER2-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer whose disease has progressed after the following in any setting: a CDK 4/6 inhibitor, endocrine therapy, and a taxane; patients received at least two prior chemotherapies in the metastatic setting (one of which could be in the neoadjuvant or adjuvant setting if recurrence occurred within 12 months).
Patients were randomized (1:1) to receive TRODELVY 10 mg/kg as an intravenous infusion on Days 1 and 8 of a 21 day cycle (n=272) or single agent chemotherapy (n=271). Single agent chemotherapy was determined by the investigator before randomization from one of the following choices: eribulin (n=130), vinorelbine (n=63), gemcitabine (n=56), or capecitabine (n=22). Randomization was stratified by the following factors: prior chemotherapy regimens for metastatic disease (2 vs. 3–4), visceral metastasis (Yes or No), and endocrine therapy in the metastatic setting for at least 6 months (Yes or No).
Patients were treated until disease progression or unacceptable toxicity. Administration of TRODELVY was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered by the investigator to be deriving clinical benefit. The primary efficacy outcome measure was PFS as determined by BICR per RECIST v1.1. Additional efficacy measures included OS, ORR by BICR, and DOR by BICR.
The median age of patients in the study population was 56 years (range: 27–86 years), 26% of patients were 65 years or over. The majority of patients were female (99%); 67% were White, 4% were Black and 3% were Asian, and 26% were of unknown race. Patients received a median of 7 (range: 3 to 17) prior systemic regimens in any setting and 3 (range: 0 to 8) prior systemic chemotherapy regimens in the metastatic setting. Approximately 42% of patients had 2 prior chemotherapy regimens for treatment of metastatic disease compared to 58% of patients who had 3 to 4 prior chemotherapy regimens and all patients had an ECOG performance status of 0 (45%) or 1 (55%). Ninety-five percent of patients had visceral metastases. Most patients received endocrine therapy in the metastatic setting for ≥ 6 months (86%).
TRODELVY demonstrated a statistically significant improvement in PFS and OS versus single agent chemotherapy.
The efficacy results are summarized in Table 12 and Figure 3 and Figure 4.
| All Randomized Patients | ||
|---|---|---|
| TRODELVY n=272 | Single Agent Chemotherapy n=271 | |
| Progression-Free Survival by BICR PFS is defined as the time from the date of randomization to the date of the first radiological disease progression or death due to any cause, whichever comes first. | ||
| Median PFS in months (95% CI) | 5.5 (4.2, 7.0) | 4.0 (3.1, 4.4) |
| Hazard ratio (95% CI) | 0.661 (0.529, 0.826) | |
| p-value Stratified log-rank test adjusted for stratification factors: prior chemotherapy regimens for metastatic disease (2 vs. 3–4), visceral metastasis (Y/N), and endocrine therapy in the metastatic setting for at least 6 months (Yes or No). BICR = Blinded Independent Central Review; CI = Confidence Interval | 0.0003 | |
| Overall Survival Second interim OS analysis (conducted when 390 OS events were observed) | ||
| Median OS in months (95% CI) | 14.4 (13.0, 15.7) | 11.2 (10.1, 12.7) |
| Hazard ratio (95% CI) | 0.789 (0.646, 0.964) | |
| p-value | 0.0200 | |
| Objective Response Rate by BICR | ||
| Response Rate, % (95% CI) | 21.0 (16.3, 26.3) | 14.0 (10.1, 18.7) |
| Odds ratio (95% CI) | 1.625 (1.034, 2.555) | |
| p-value | 0.0348 | |
| Duration of Response (DOR) by BICR | ||
| Median DOR in months (95% CI) | 8.1 (6.7, 9.1) | 5.6 (3.8, 7.9) |
Figure 3: Kaplan-Meier Plot of PFS by BICR in TROPiCS-02
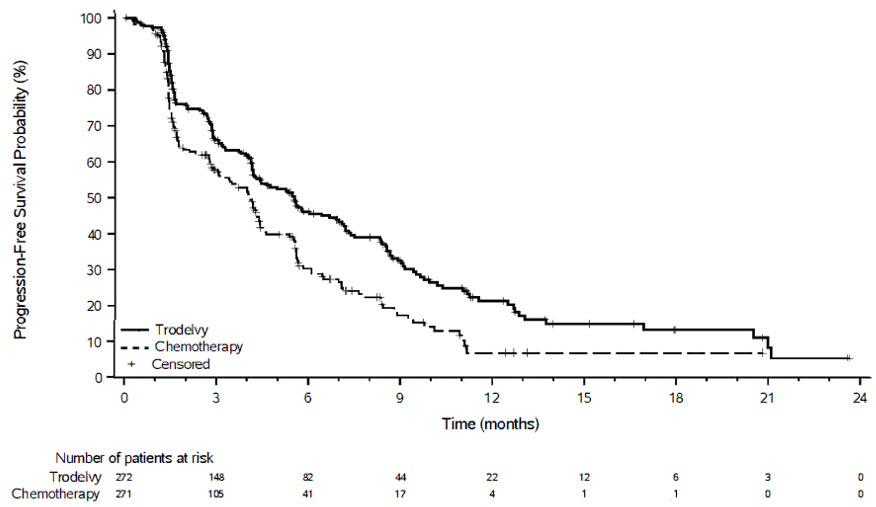
Figure 4: Kaplan-Meier Plot of OS in TROPiCS-02
HOW SUPPLIED/STORAGE AND HANDLING
TRODELVY (sacituzumab govitecan-hziy) for injection is a sterile, off-white to yellowish lyophilized powder in a single-dose vial. Each TRODELVY vial is individually boxed in a carton:
- NDC 55135-132-01 contains one 180 mg vial
Store vials in a refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light until time of reconstitution. Do not freeze.
TRODELVY is a hazardous drug. Follow applicable special handling and disposal procedures 1 .
Mechanism of Action
Sacituzumab govitecan-hziy is a Trop-2-directed antibody-drug conjugate. Sacituzumab is a humanized antibody that recognizes Trop-2. The small molecule, SN-38, is a topoisomerase I inhibitor, which is covalently attached to the antibody by a linker. Pharmacology data suggest that sacituzumab govitecan-hziy binds to Trop-2-expressing cancer cells and is internalized with the subsequent release of SN-38 via hydrolysis of the linker. SN-38 interacts with topoisomerase I and prevents re-ligation of topoisomerase I-induced single strand breaks. The resulting DNA damage leads to apoptosis and cell death. Sacituzumab govitecan-hziy decreased tumor growth in mouse xenograft models of triple-negative breast cancer.