Twirla prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Twirla patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
2.1 How to Start Using TWIRLA
See the FDA-approved patient labeling (Instructions for Use ) .
The TWIRLA transdermal system (TDS) is used in a 28-day (four-week) cycle. A new TDS is applied and worn for seven days for three consecutive weeks (Weeks 1, 2, and 3). No TDS is worn during Week 4 (the TDS-Free Week), when withdrawal bleeding is expected.
On the day after Week 4 ends, a new 28-day cycle is started by applying a new TDS. Under no circumstances should there be more than a 7-day TDS-free interval between dosing cycles.
Breakthrough (Unscheduled) Bleeding or Spotting Occurrence
If unscheduled (breakthrough) spotting or bleeding occurs, instruct the woman to continue the same regimen. If the bleeding is persistent or prolonged consider causes other than TWIRLA. If the bleeding is persistent or prolonged, instruct the woman to consult with her healthcare provider.
In Case of Skin Irritation
If TDS use results in uncomfortable irritation, the TDS may be removed, and a new TDS may be applied to a different location until the next “Patch Change Day”. Only one TDS should be worn at a time.
Every new TDS should be applied on the same day of the week. This day is known as the “Patch Change Day.” For example, if the first TDS is applied on a Sunday, all subsequent TDS should be applied on a Sunday.
There are multiple options for starting the TDS, and the woman should choose the option that is most appropriate (see Table 1):
Starting TWIRLA in women with no current use of hormonal contraception | Day 1 Start
|
Switching from another contraceptive method
| Start TWIRLA:
|
|
|
|
|
|
|
|
|
|
|
|
|
Use after an abortion or miscarriage:
TWIRLA may be started immediately for contraception within the first 5 days following a complete first trimester abortion or miscarriage without additional back-up contraception. If more than 5 days have elapsed from the first trimester abortion or miscarriage, then the woman should be advised to use non-hormonal contraception (such as condoms and spermicide, or diaphragm and spermicide) and follow instructions for starting TWIRLA for the first time. Ovulation may occur within 10 days of an abortion or miscarriage.
TWIRLA should not be started earlier than 4 weeks after a second trimester abortion or miscarriage due to the increased risk of thromboembolism [see Warnings and Precautions (5.1 )] .
Use of TWIRLA after childbirth:
For women who elect not to breastfeed, do not start TWIRLA sooner than 4 weeks after childbirth given the increased risk for thromboembolism [see Use in Specific Populations (8.2 )] .
If a woman begins using TWIRLA postpartum and has not yet had a period, consider the possibility of ovulation and pregnancy. If the woman is not pregnant, instruct her to use non-hormonal back-up contraception (such as condoms and spermicide, or diaphragm and spermicide) for the first 7 days of TDS use [see Warnings and Precautions (5.1 ) and Use in Specific Populations (8.1 )] .
2.2 Important Application Instructions
- See the FDA-approved patient labeling (Instructions for Use ) .
- TWIRLA TDS is applied once weekly for three weeks. Each TWIRLA TDS should be worn for one week. Instruct women to wear only one TWIRLA TDS at any time.
- To achieve maximum contraceptive effectiveness, TWIRLA must be used exactly as directed. The failure rate may increase when TDS application is delayed/missed or when TDS is applied incorrectly.
- Apply TWIRLA to clean, dry, and intact skin at the selected application site. Application sites include: the abdomen, buttock or upper torso (excluding the breasts). When applying a new TWIRLA TDS, do not apply the new TDS directly over the previous TDS site.
- Do not apply TWIRLA to skin that has been exposed to powder, oil, moisturizer, or lotion. Advise women not to routinely use large amounts of body lotions or oils at application sites.
- Prolonged exposure to water may interfere with adherence of TWIRLA.
- Do not cut or alter TWIRLA in any way, the whole TDS should be applied. If the TWIRLA TDS is cut or damaged or altered in size, contraceptive efficacy may be impaired.
- If the TWIRLA TDS lifts at the edges, reattach TWIRLA by pressing firmly and smoothing down the edges of the system. If TWIRLA comes off completely, reapply the TWIRLA TDS that detached.
- Discard TWIRLA by folding the used TDS so that the adhesive side sticks to itself and safely discard in the trash.
MANAGING PARTIAL OR COMPLETE TDS DETACHMENTS (see Table 2 )
The TWIRLA TDS must adhere securely to the skin to work properly. Prolonged water exposure may compromise the TDS’s adherence. As a result, the woman should be instructed to check the TDS for partial or complete TDS detachment not only daily but also after prolonged water exposure.
If the TDS becomes partially or completely detached and remains detached, insufficient drug delivery may occur. Partial TDS detachment should be resolved since it can lead to the TDS getting caught on clothing and detaching. The woman should not try to reapply a TDS if it is no longer sticky, if it has become stuck to itself or another surface, and/or if it has other material stuck to it.
If a TDS edge lifts up:
- The woman should press down firmly on the TDS with the palm of the hand for 10 seconds, making sure that the whole TDS is adhered to her skin. Then run fingers over the entire surface area to smooth out any wrinkles around the edges of the TDS.
- If the lifted edge of the TDS does not stick completely after attempted re-adhesion, the TDS should be removed, and a new replacement TDS applied.
- Do not tape or wrap the TDS to the skin or reapply a TDS that is partially adhered to clothing.
If the TDS has been off or partially off:
- For less than one day , the woman should try to reapply it. If the TDS does not adhere completely, apply a new TDS immediately. No back-up contraception is needed and the “Patch Change Day” will stay the same.
- For more than one day OR if unsure of the timeframe , the woman may not be protected from pregnancy. To reduce this risk, apply a new TDS and start a new 4-week cycle. The woman will now have a new “Patch Change Day” and MUST USE NON-HORMONAL BACK-UP CONTRACEPTION (such as condoms and spermicide, or diaphragm and spermicide) for the first 7 days of the new cycle.
Missed Doses
Instruct women about the handling of missed doses (e.g., missed or delayed TDS application) and to follow the dosing instructions provided in the FDA-approved patient labeling.
FORGETTING TO CHANGE THE TDS:
- At the start of any TDS cycle (Week 1/Day 1): THE WOMAN MAY NOT BE PROTECTED FROM PREGNANCY. The woman should apply the first TDS of her new cycle as soon as she remembers, and this becomes the new "Patch Change Day" and a new "Day 1" of the cycle. The woman should use non-hormonal back-up contraception (such as condoms and spermicide, or diaphragm and spermicide) for the first 7 days of the new cycle.
- In the middle of the TDS cycle (Week 2/Day 8 or Week 3/Day 15), for 1 or 2 days (up to 48 hours): The woman should apply a new TDS immediately. The next TDS should be applied on the usual "Patch Change Day". No back-up contraception is needed.
- For more than 2 days (48 hours or more): THE WOMAN MAY NOT BE PROTECTED FROM PREGNANCY. The woman should stop the current contraceptive cycle and start a new four-week cycle immediately by putting on a new TDS. This is now a new "Patch Change Day" and a new "Day 1" of the cycle. Non-hormonal back-up contraception must be used for 7 days.
- At the end of the TDS cycle Week 3 (Day 22): If the woman forgets to remove her TDS, she should take it off as soon as she remembers. The next cycle should be started on the usual "Patch Change Day", which is the day after Day 28. No back-up contraception is needed.
| Scenario | Results in New TDS- Change Day | Back-up Contraception Required (7 Days) | Starts New Cycle |
| Did not apply TDS on scheduled Day 1/Week 1 of new cycle (late TDS-on day) | Yes | Yes | Yes |
| TDS detached for < 24 hours | No | No | No |
| TDS detached for ≥ 24 hours, or unsure duration | Yes | Yes | Yes |
| < 48 hours late for Patch Change Day (Day 8 or 15) | No | No | No |
| ≥ 48 hours late for Patch Change Day (Day 8 or 15) | Yes | Yes | Yes |
| Forgets to remove last TDS on Day 22 | No | No | No |
Under no circumstances should there be more than a seven-day TDS-free interval between cycles. If there are more than 7 TDS-free days, THE WOMAN MAY NOT BE PROTECTED FROM PREGNANCY and non-hormonal back-up contraception (such as a condoms and spermicide, or diaphragm and spermicide) must be used for 7 days. As with CHCs, the risk of ovulation increases with each day beyond the recommended drug-free period. If the woman has intercourse during such an extended TDS-free interval, consider the possibility of pregnancy.

By using PrescriberAI, you agree to the AI Terms of Use.
Twirla prescribing information
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS and CONTRAINDICATED IN WOMEN WITH A BMI ≥ 30 KG/M 2
Cigarette Smoking and Serious Cardiovascular Events Cigarette smoking increases the risk of serious cardiovascular events from combined hormonal contraceptive (CHC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, CHCs, including TWIRLA, are contraindicated in women who are over 35 years of age and smoke [see Contraindications (4 ) and Warnings and Precautions (5.1 )].
Contraindicated in Women with a BMI ≥ 30 kg/m 2 TWIRLA is contraindicated in women with a BMI ≥ 30 kg/m 2 . Compared to women with a lower BMI, women with a BMI ≥ 30 kg/m 2 had reduced effectiveness and may have a higher risk for venous thromboembolism events (VTEs) [see Contraindications (4 ) and Warnings and Precautions (5.1 )] .
INDICATIONS AND USAGE
TWIRLA is indicated as a method of contraception for use in women of reproductive potential with a BMI < 30 kg/m 2 for whom a combined hormonal contraceptive is appropriate.
Limitation of Use Consider TWIRLA’s reduced effectiveness in women with a BMI ≥ 25 to < 30 kg/m 2 before prescribing TWIRLA [see Use in Specific Populations (8.9 ) and Clinical Studies (14 )] . TWIRLA is contraindicated in women with a BMI ≥ 30 kg/m 2 [see Contraindications (4 )] .
DOSAGE AND ADMINISTRATION
2.1 How to Start Using TWIRLA
See the FDA-approved patient labeling (Instructions for Use ) .
The TWIRLA transdermal system (TDS) is used in a 28-day (four-week) cycle. A new TDS is applied and worn for seven days for three consecutive weeks (Weeks 1, 2, and 3). No TDS is worn during Week 4 (the TDS-Free Week), when withdrawal bleeding is expected.
On the day after Week 4 ends, a new 28-day cycle is started by applying a new TDS. Under no circumstances should there be more than a 7-day TDS-free interval between dosing cycles.
Breakthrough (Unscheduled) Bleeding or Spotting Occurrence If unscheduled (breakthrough) spotting or bleeding occurs, instruct the woman to continue the same regimen. If the bleeding is persistent or prolonged consider causes other than TWIRLA. If the bleeding is persistent or prolonged, instruct the woman to consult with her healthcare provider.
In Case of Skin Irritation If TDS use results in uncomfortable irritation, the TDS may be removed, and a new TDS may be applied to a different location until the next “Patch Change Day”. Only one TDS should be worn at a time.
Every new TDS should be applied on the same day of the week. This day is known as the “Patch Change Day.” For example, if the first TDS is applied on a Sunday, all subsequent TDS should be applied on a Sunday.
There are multiple options for starting the TDS, and the woman should choose the option that is most appropriate (see Table 1):
Starting TWIRLA in women with no current use of hormonal contraception | Day 1 Start
|
Switching from another contraceptive method
| Start TWIRLA:
|
|
|
|
|
|
|
|
|
|
|
|
|
Use after an abortion or miscarriage: TWIRLA may be started immediately for contraception within the first 5 days following a complete first trimester abortion or miscarriage without additional back-up contraception. If more than 5 days have elapsed from the first trimester abortion or miscarriage, then the woman should be advised to use non-hormonal contraception (such as condoms and spermicide, or diaphragm and spermicide) and follow instructions for starting TWIRLA for the first time. Ovulation may occur within 10 days of an abortion or miscarriage.
TWIRLA should not be started earlier than 4 weeks after a second trimester abortion or miscarriage due to the increased risk of thromboembolism [see Warnings and Precautions (5.1 )] .
Use of TWIRLA after childbirth: For women who elect not to breastfeed, do not start TWIRLA sooner than 4 weeks after childbirth given the increased risk for thromboembolism [see Use in Specific Populations (8.2 )] .
If a woman begins using TWIRLA postpartum and has not yet had a period, consider the possibility of ovulation and pregnancy. If the woman is not pregnant, instruct her to use non-hormonal back-up contraception (such as condoms and spermicide, or diaphragm and spermicide) for the first 7 days of TDS use [see Warnings and Precautions (5.1 ) and Use in Specific Populations (8.1 )] .
2.2 Important Application Instructions
- See the FDA-approved patient labeling (Instructions for Use ) .
- TWIRLA TDS is applied once weekly for three weeks. Each TWIRLA TDS should be worn for one week. Instruct women to wear only one TWIRLA TDS at any time.
- To achieve maximum contraceptive effectiveness, TWIRLA must be used exactly as directed. The failure rate may increase when TDS application is delayed/missed or when TDS is applied incorrectly.
- Apply TWIRLA to clean, dry, and intact skin at the selected application site. Application sites include: the abdomen, buttock or upper torso (excluding the breasts). When applying a new TWIRLA TDS, do not apply the new TDS directly over the previous TDS site.
- Do not apply TWIRLA to skin that has been exposed to powder, oil, moisturizer, or lotion. Advise women not to routinely use large amounts of body lotions or oils at application sites.
- Prolonged exposure to water may interfere with adherence of TWIRLA.
- Do not cut or alter TWIRLA in any way, the whole TDS should be applied. If the TWIRLA TDS is cut or damaged or altered in size, contraceptive efficacy may be impaired.
- If the TWIRLA TDS lifts at the edges, reattach TWIRLA by pressing firmly and smoothing down the edges of the system. If TWIRLA comes off completely, reapply the TWIRLA TDS that detached.
- Discard TWIRLA by folding the used TDS so that the adhesive side sticks to itself and safely discard in the trash.
MANAGING PARTIAL OR COMPLETE TDS DETACHMENTS (see Table 2 ) The TWIRLA TDS must adhere securely to the skin to work properly. Prolonged water exposure may compromise the TDS’s adherence. As a result, the woman should be instructed to check the TDS for partial or complete TDS detachment not only daily but also after prolonged water exposure.
If the TDS becomes partially or completely detached and remains detached, insufficient drug delivery may occur. Partial TDS detachment should be resolved since it can lead to the TDS getting caught on clothing and detaching. The woman should not try to reapply a TDS if it is no longer sticky, if it has become stuck to itself or another surface, and/or if it has other material stuck to it.
If a TDS edge lifts up:
- The woman should press down firmly on the TDS with the palm of the hand for 10 seconds, making sure that the whole TDS is adhered to her skin. Then run fingers over the entire surface area to smooth out any wrinkles around the edges of the TDS.
- If the lifted edge of the TDS does not stick completely after attempted re-adhesion, the TDS should be removed, and a new replacement TDS applied.
- Do not tape or wrap the TDS to the skin or reapply a TDS that is partially adhered to clothing.
If the TDS has been off or partially off:
- For less than one day , the woman should try to reapply it. If the TDS does not adhere completely, apply a new TDS immediately. No back-up contraception is needed and the “Patch Change Day” will stay the same.
- For more than one day OR if unsure of the timeframe , the woman may not be protected from pregnancy. To reduce this risk, apply a new TDS and start a new 4-week cycle. The woman will now have a new “Patch Change Day” and MUST USE NON-HORMONAL BACK-UP CONTRACEPTION (such as condoms and spermicide, or diaphragm and spermicide) for the first 7 days of the new cycle.
Missed Doses
Instruct women about the handling of missed doses (e.g., missed or delayed TDS application) and to follow the dosing instructions provided in the FDA-approved patient labeling.
FORGETTING TO CHANGE THE TDS:
- At the start of any TDS cycle (Week 1/Day 1): THE WOMAN MAY NOT BE PROTECTED FROM PREGNANCY. The woman should apply the first TDS of her new cycle as soon as she remembers, and this becomes the new "Patch Change Day" and a new "Day 1" of the cycle. The woman should use non-hormonal back-up contraception (such as condoms and spermicide, or diaphragm and spermicide) for the first 7 days of the new cycle.
- In the middle of the TDS cycle (Week 2/Day 8 or Week 3/Day 15), for 1 or 2 days (up to 48 hours): The woman should apply a new TDS immediately. The next TDS should be applied on the usual "Patch Change Day". No back-up contraception is needed.
- For more than 2 days (48 hours or more): THE WOMAN MAY NOT BE PROTECTED FROM PREGNANCY. The woman should stop the current contraceptive cycle and start a new four-week cycle immediately by putting on a new TDS. This is now a new "Patch Change Day" and a new "Day 1" of the cycle. Non-hormonal back-up contraception must be used for 7 days.
- At the end of the TDS cycle Week 3 (Day 22): If the woman forgets to remove her TDS, she should take it off as soon as she remembers. The next cycle should be started on the usual "Patch Change Day", which is the day after Day 28. No back-up contraception is needed.
| Scenario | Results in New TDS- Change Day | Back-up Contraception Required (7 Days) | Starts New Cycle |
| Did not apply TDS on scheduled Day 1/Week 1 of new cycle (late TDS-on day) | Yes | Yes | Yes |
| TDS detached for < 24 hours | No | No | No |
| TDS detached for ≥ 24 hours, or unsure duration | Yes | Yes | Yes |
| < 48 hours late for Patch Change Day (Day 8 or 15) | No | No | No |
| ≥ 48 hours late for Patch Change Day (Day 8 or 15) | Yes | Yes | Yes |
| Forgets to remove last TDS on Day 22 | No | No | No |
Under no circumstances should there be more than a seven-day TDS-free interval between cycles. If there are more than 7 TDS-free days, THE WOMAN MAY NOT BE PROTECTED FROM PREGNANCY and non-hormonal back-up contraception (such as a condoms and spermicide, or diaphragm and spermicide) must be used for 7 days. As with CHCs, the risk of ovulation increases with each day beyond the recommended drug-free period. If the woman has intercourse during such an extended TDS-free interval, consider the possibility of pregnancy.
DOSAGE FORMS AND STRENGTHS
TWIRLA (120 mcg/day levonorgestrel and 30 mcg/day ethinyl estradiol) transdermal system is a circular beige colored product with the name and strength etched on the backing membrane.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary TWIRLA is contraindicated in pregnancy because there is no reason to use CHCs in pregnancy. Discontinue TWIRLA if pregnancy occurs. Epidemiologic studies and meta-analyses have not found an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to CHCs before conception or during early pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Lactation
Risk Summary Contraceptive hormones and/or metabolites are present in human milk. CHCs can reduce milk production in breastfeeding women. This reduction can occur at any time but is less likely to occur once breastfeeding is well established. Advise the nursing woman to use another method of contraception until she discontinues breastfeeding [see Dosage and Administration (2.1 )] .
Human Data No studies have been conducted on the use of TWIRLA in breastfeeding women.
Pediatric Use
The safety and effectiveness of TWIRLA as a method of contraception have been established in females of reproductive potential with a BMI < 30 kg/m 2 . Efficacy is expected to be the same in postmenarcheal females regardless of age. TWIRLA is not indicated in females before menarche.
Geriatric Use
TWIRLA has not been studied in postmenopausal women and is not indicated in this population.
Hepatic Impairment
No studies have been conducted to evaluate the effect of hepatic impairment on the disposition of TWIRLA. However, steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of CHC use until markers of liver function return to normal and CHC causation has been excluded [see Contraindications (4 ) and Warnings and Precautions (5.2 )].
Body Mass Index (BMI)
Compared to women with a lower BMI, women with a BMI ≥ 30 kg/m 2 had reduced effectiveness and may have a higher risk for VTEs. Therefore, TWIRLA is contraindicated in women with a BMI ≥ 30 kg/m 2 [see Contraindications (4 ) and Clinical Studies (14 )] .
TWIRLA has demonstrated reduced efficacy in women with a BMI ≥ 25 and < 30 kg/m 2 [see Clinical Studies (14 )] . Consider this before prescribing TWIRLA to women with a BMI ≥ 25 to < 30 kg/m 2 .
CONTRAINDICATIONS
Twirla is contraindicated in females who are known to have or develop the following conditions:
- At high risk of arterial or venous thrombotic diseases. Examples include women who
- Smoke, if over age 35 [see Boxed Warning and Warnings and Precautions (5.1 )]
- Have current or history of deep vein thrombosis or pulmonary embolism [see Warnings and Precautions (5.1 )]
- Have cerebrovascular disease [see Warnings and Precautions (5.1 )]
- Have coronary artery disease [see Warnings and Precautions (5.1 )]
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see Warnings and Precautions (5.1 )]
- Have inherited or acquired hypercoagulopathies [see Warnings and Precautions (5.1 )]
- Have uncontrolled hypertension or hypertension with vascular disease [see Warnings and Precautions (5.4 )]
- Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration [see Warnings and Precautions (5.7 )]
- Have headaches with focal neurological symptoms, migraine headaches with aura
- Women over age 35 with any migraine headaches [see Warnings and Precautions (5.8 )]
- BMI ≥ 30 kg/m 2 . Compared to women with a lower BMI, women with a BMI ≥ 30 kg/m 2 had reduced effectiveness and may have a higher risk for VTEs [see Warnings and Precautions (5.1 ), Use in Specific Populations (8.9 ) and Clinical Studies (14 )].
- Liver tumors (benign or malignant), acute viral hepatitis, or severe (decompensated) cirrhosis, or liver disease [see Warnings and Precautions (5.2 )]
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.9 )]
- Pregnancy, because there is no reason to use CHCs during pregnancy [see Use in Specific Populations (8.1 )]
- Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive [see Warnings and Precautions (5.11 )]
- Hypersensitivity to any components of TWIRLA. Observed reactions include itching and irritation at the TDS application site [see Adverse Reactions (6.1 )]
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for alanine aminotransferase (ALT) elevations [see Warnings and Precautions (5.3 )]
WARNINGS AND PRECAUTIONS
Thromboembolic Disorders and Other Vascular Conditions
Women are at increased risk for a venous thromboembolic event (VTE) when using CHCs, including TWIRLA. The risk of VTE may be greater in women with a BMI ≥ 30 kg/m 2 compared to women with a lower BMI, and TWIRLA is contraindicated in obese patients [see Contraindications (4 )] . In the Phase 3 clinical trial, four TWIRLA-treated women experienced a VTE. All of these women had a BMI > 30 kg/m 2 [see Adverse Reactions (6.1 )] .
- Stop TWIRLA if an arterial or venous thromboembolic event occurs.
- Stop TWIRLA if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
- Discontinue TWIRLA during prolonged immobilization and resume treatment based on clinical judgement. If feasible, stop TWIRLA at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
- Start TWIRLA no earlier than four weeks after delivery in women who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.
- Before starting TWIRLA, evaluate any past medical history or family history of thromboembolism or thromboembolic disorders. Consider whether the history suggests an inherited or acquired hypercoagulopathy. TWIRLA is contraindicated in women with a high risk of arterial or venous thromboembolic diseases [see Contraindications (4 )] .
Arterial Events CHCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and women with hypertension, dyslipidemia, diabetes, or obesity.
TWIRLA is contraindicated in women over 35 years of age who smoke [see Contraindications (4 ) ] . Cigarette smoking increases the risk of serious cardiovascular events from CHC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous Events The use of CHCs increases the risk of VTEs, such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs. While the increased risk of VTE associated with the use of CHCs is well-established, the rates of VTE are even greater during pregnancy, especially during the postpartum period (see Figure 1 ). The rate of VTE in women using CHCs has been estimated to be 3 to 12 cases per 10,000 woman-years for non-oral CHCs.
The risk of VTE is highest during the first year of use of a combined oral contraceptive (COC) and when restarting hormonal contraception after a break of four weeks or longer. This initial higher risk declines during the first year, but users of CHCs remain at an increased risk of VTE compared to non-users of CHCs. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to CHCs gradually disappears after CHC use is discontinued.
Figure 1 shows the risk of developing a VTE for women who are not pregnant and do not use hormonal contraceptives, for women who use hormonal contraceptives with a range of doses and routes of administration, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use hormonal contraceptives are followed for one year, between 1 and 5 of these women will develop a VTE.
Figure 1. Likelihood of Developing a VTE Within One Year Among Pregnant and Non-Pregnant Women
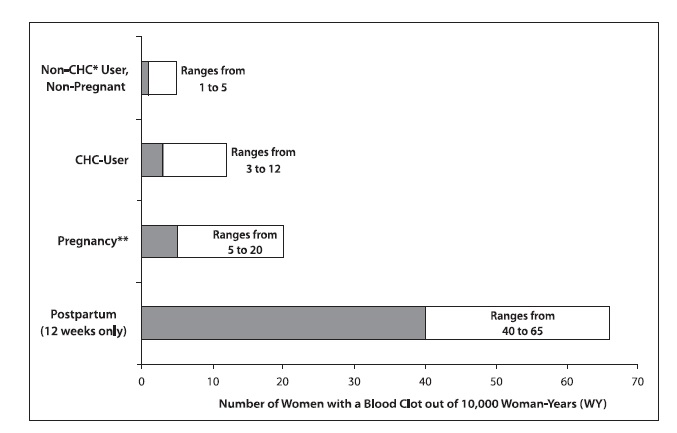 •CHC = combination hormonal contraception •• Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is 9 months, the rate is 7 to 27 per 10,000 WY.
•CHC = combination hormonal contraception •• Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is 9 months, the rate is 7 to 27 per 10,000 WY.
Liver Disease
Elevated Liver Enzymes TWIRLA is contraindicated in women with acute viral hepatitis or severe (decompensated) cirrhosis of the liver [see Contraindications (4 )] . Discontinue TWIRLA if jaundice develops. Acute liver test abnormalities may necessitate the discontinuation of CHC use until the liver tests return to normal and CHC causation has been excluded.
Liver Tumors TWIRLA is contraindicated in women with benign or malignant liver tumors [see Contraindications (4 )] . CHCs increase the risk of hepatic adenomas. An estimate of the attributable risk is 3.3 cases/100,000 CHC users. Rupture of hepatic adenomas may cause death from abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (> 8 years) CHC users. The attributable risk of liver cancers in CHC users is less than one case per million users.
5.3 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as CHCs. CHCs, such as TWIRLA, are contraindicated for use with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications (4 )] . Discontinue TWIRLA prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. TWIRLA can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.
Hypertension
TWIRLA is contraindicated in women with uncontrolled hypertension or hypertension with vascular disea se [see Contraindications (4 )] . For all women, including those with well-controlled hypertension, monitor blood pressure at routine visits and stop TWIRLA if blood pressure rises significantly.
An increase in blood pressure has been reported in women using CHCs, and this increase is more likely in older women with extended duration of use. The effect of CHCs on blood pressure may vary according to the progestin in the CHC.
5.5 Age-Related Considerations
The risk for cardiovascular disease and prevalence of risk factors for cardiovascular disease increase with age. Certain conditions, such as smoking and migraine headache without aura, that do not contraindicate CHC use in younger women, are contraindications to use in women over 35 years of age [see Contraindications (4 ) and Warnings and Precautions (5.1 )] . Consider the presence of underlying risk factors that may increase the risk of cardiovascular disease or VTE, particularly before initiating a CHC for women over 35 years, such as:
- Hypertension
- Diabetes
- Dyslipidemia
- Obesity
5.6 Gallbladder Disease
Studies suggest an increased risk of developing gallbladder disease among CHC users. Use of CHCs may also worsen existing gallbladder disease.
A past history of CHC-related cholestasis predicts an increased risk with subsequent CHC use. Women with a history of pregnancy-related cholestasis may be at an increased risk for CHC-related cholestasis.
Adverse Carbohydrate and Lipid Metabolic Effects
Hyperglycemia TWIRLA is contraindicated in diabetic women over age 35, or women who have diabetes with hypertension, nephropathy, retinopathy, neuropathy, other vascular disease, or women with diabetes of > 20 years duration [see Contraindications (4 )] . TWIRLA may decrease glucose tolerance. Carefully monitor prediabetic and diabetic women who are using TWIRLA.
Dyslipidemia Consider alternative contraception for women with uncontrolled dyslipidemia. TWIRLA may cause adverse lipid changes.
Women with hypertriglyceridemia, or a family history thereof, may have an increase in serum triglyceride concentrations when using TWIRLA, which may increase the risk of pancreatitis.
Headache
TWIRLA is contraindicated in women who have headaches with focal neurological symptoms or have migraine headaches with aura, and in women over age 35 years who have migraine headaches with or without aura [see Contraindications (4 )] .
If a woman using TWIRLA develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue TWIRLA if indicated. Consider discontinuation of TWIRLA if there is any increased frequency or severity of migraines during CHC use (which may be prodromal of a cerebrovascular event).
Bleeding Irregularities and Amenorrhea
Unscheduled and Scheduled Bleeding and Spotting Women using TWIRLA may experience unscheduled (breakthrough or intracyclic) bleeding and spotting, especially during the first three months of use. Bleeding irregularities may resolve over time or by changing to a different contraceptive product. If bleeding persists or occurs after previously regular cycles on TWIRLA, evaluate for causes such as pregnancy or malignancy.
Based on women’s electronic diaries from a clinical trial evaluating the safety and efficacy of TWIRLA, the proportion of subjects reporting unscheduled bleeding per 28-day cycle decreased over time. At cycle 1 and 2, 60.4% and 52.6%, respectively reported unscheduled bleeding and/or spotting. At cycle 13, 42.3% of women reported unscheduled bleeding and/or spotting. Women reported a mean number of unscheduled bleeding/spotting days per month that generally decreased over the 13 cycles and was a mean of 1.6 days in Cycle 13. A total of 45 women (2.2%) discontinued the study prematurely due to menstrual disorders including metrorrhagia, vaginal hemorrhage, menorrhagia, dysmenorrhea, irregular menstruation, dysfunctional uterine bleeding, and menstrual disorder [see Clinical Trial Experience (6.1 ) and Clinical Studies (14 )] .
Amenorrhea and Oligomenorrhea Women who use TWIRLA may experience absence of scheduled (withdrawal) bleeding, even if they are not pregnant. Based on electronic patient diaries from the clinical trial, the percentages of women with no bleeding and/or spotting days (amenorrhea) in a cycle ranged from 6.3% to 11.9% over 13 cycles [see Clinical Trial Experience (6.1 ) and Clinical Studies (14 )] .
If scheduled bleeding does not occur, consider the possibility of pregnancy. If the woman has not adhered to the prescribed dosing schedule (missed days of active therapy or started her TDS on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and perform appropriate diagnostic measures. If the woman has adhered to the prescribed dosing schedule and misses two consecutive periods, rule out pregnancy.
After discontinuation of TWIRLA, amenorrhea or oligomenorrhea may occur, especially if these conditions were pre-existent.
Depression
Carefully observe women with a history of depression and discontinue TWIRLA if depression recurs to a serious degree. Data on the association of CHCs with onset of depression or exacerbation of existing depression are limited.
5.11 Malignant Neoplasms
Breast Cancer Twirla is contraindicated in females who currently have or have had breast cancer because breast cancer may be hormonally sensitive [see Contraindications (4 )] .
Epidemiology studies have not found a consistent association between use of COCs and breast cancer risk. Studies do not show an association between ever (current or past) use of COCs and risk of breast cancer. However, some studies report a small increase in the risk of breast cancer among current or recent users (<6 months since last use) and current users with longer duration of COC use [see Postmarketing Experience (6.2 )].
Cervical Cancer Some studies suggest that CHCs are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. There is controversy about the extent to which these findings are due to differences in sexual behavior and other factors.
Effect on Binding Globulins
The estrogen component of TWIRLA may raise the serum concentrations of thyroxine-binding globulin, sex hormone-binding globulin, and cortisol-binding globulin. The dose of replacement thyroid hormone or cortisol therapy may need to be increased.
Hereditary Angioedema
In women with hereditary angioedema, exogenous estrogens may induce or exacerbate symptoms of angioedema.
Chloasma
Chloasma may occur with TWIRLA use, especially in women with a history of chloasma gravidarum. Advise women with a history of chloasma to avoid exposure to the sun or ultraviolet radiation while using TWIRLA.
ADVERSE REACTIONS
The following serious adverse reactions with the use of CHCs, including TWIRLA, are discussed elsewhere in the labeling:
Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of one product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in practice.
The safety of TWIRLA was evaluated in a 12-month, multicenter, open-label, single-arm clinical trial (NCT02158572) conducted in the United States [see Clinical Studies (14 )] . Women applied TWIRLA (120 mcg LNG/30 mcg EE) for 13 28-day treatment cycles. One treatment cycle is defined as three consecutive weeks that one TWIRLA TDS is applied for seven-day wear followed by one week that TWIRLA is not applied.
The safety population for this clinical trial was composed of 2,031 women that contributed 18,841 treatment cycles of exposure. Of these 2,031 women, 989 women completed 13 treatment cycles. The mean age was 27.5 years. The mean BMI for the safety population was 28.3 kg/m 2 . The BMI of the safety population was widely distributed: 39.4% had a BMI < 25 kg/m 2 , 25.3% had a BMI ≥ 25 kg/m 2 and < 30 kg/m 2 , and 35.3% had a BMI ≥ 30 kg/m 2 .
For women who received TWIRLA, the most common reasons for discontinuation from the study were a womans decision (15.3%) and lost to follow-up (11.3%).
Discontinuation due to an adverse reaction occurred in 10.9% of women. The most common (≥ 2%) adverse reactions leading to discontinuation were application site disorder (3.1%) and any bleeding irregularities (2.2%).
The most common adverse reactions that occurred in ≥ 2% of the 2,031 women that used TWIRLA are shown in Table 3.
| Adverse reaction | TWIRLA (n=2,031) |
| General disorders and administration site conditions Application site disorder Represents a bundle of similar terms that include the following adverse reactions: application site acne, hemorrhage, pustules, dermatitis, hypersensitivity, rash, discoloration, induration, reaction, dryness, irritation, ulcer, erosion, pain, urticaria, erythema, papules, vesicles, exfoliation, pruritis. | 6.2% |
| Gastrointestinal disorders Nausea | 4.1% |
| Nervous system disorders Headache | 3.6% |
| Reproductive system and breast disorder Dysmenorrhoea | 2.3% |
| Investigations Weight increased | 2.0% |
Venous Thromboembolic Events (VTEs)
A total of four VTEs (including pulmonary embolism and deep vein thrombosis) in TWIRLA-treated patients were identified in the clinical trial. Of these, all were in women with a BMI > 30 kg/m 2 [see Contraindications (4 )] .
Other Serious Adverse Reactions The following serious adverse reactions occurred in < 1% of women who received TWIRLA: cholelithiasis, cholecystitis, major depression, suicidal ideation, appendicitis, ectopic pregnancy, pneumonia, and gastroenteritis.
Postmarketing Experience
Five studies that compared breast cancer risk between ever-users (current or past use) of combined oral contraceptives (COCs) and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 - 1.12 (Figure 2).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 2). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 - 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.
Figure 2. Relevant Studies of Risk of Breast Cancer with Combined Oral Contraceptives
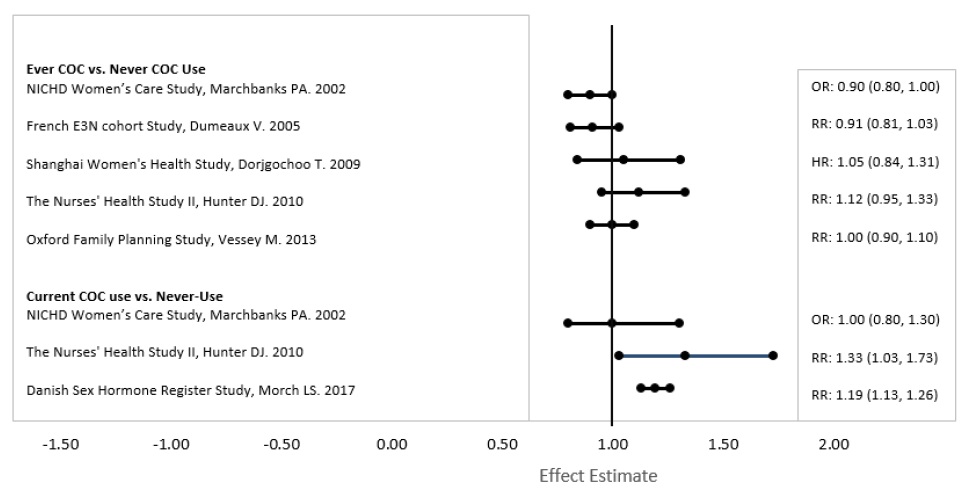
RR = relative risk; OR = odds ratio; HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are females that never used COCs.
DRUG INTERACTIONS
The sections below provide information on substances for which data on drug interactions with CHCs are available. There is little information available about the clinical effect of most drug interactions that may affect CHCs. However, based on the known pharmacokinetic effects of these drugs, clinical strategies to minimize any potential adverse effect on contraceptive effectiveness or safety are suggested.
Consult the approved product labeling of all concurrently used drugs to obtain further information about interactions with CHCs or the potential for metabolic enzyme or transporter system alterations.
No drug-drug interaction studies were conducted with TWIRLA.
Effects of Other Drugs on Combined Hormonal Contraceptives
Substances Decreasing Plasma Concentration of CHCs and Potentially Diminishing the Efficacy of CHCs:
Table 4 includes substances that demonstrated an important drug interaction with TWIRLA.
Metabolic Enzyme Inducers | |
Clinical effect |
|
Prevention or management |
|
Examples |
|
Colesevelam | |
Clinical effect |
|
Prevention or management | Administer 4 or more hours apart to attenuate this drug interaction. |
Substances increasing the systemic exposure of CHCs : Co-administration of atorvastatin or rosuvastatin and CHCs containing ethinyl estradiol increase systemic exposure of ethinyl estradiol by approximately 20 to 25 percent. Ascorbic acid and acetaminophen may increase systemic exposure of ethinyl estradiol, possibly by inhibition of conjugation. CYP3A4 inhibitors such as itraconazole, voriconazole, fluconazole, grapefruit juice, or ketoconazole may increase systemic exposure of the estrogen and/or progestin component of CHCs.
Human immunodeficiency virus (HIV)/hepatitis C virus (HCV) protease inhibitors and non-nucleoside reverse transcriptase inhibitors: Significant decreases in systemic exposure of the estrogen and/or progestin have been noted when CHCs are co-administered with some HIV protease inhibitors (e.g., nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/ritonavir, and tipranavir/ritonavir), some HCV protease inhibitors (e.g., boceprevir and telaprevir), and some non-nucleoside reverse transcriptase inhibitors (e.g., nevirapine).
In contrast, significant increases in systemic exposure of the estrogen and/or progestin have been noted when CHCs are co-administered with certain other HIV protease inhibitors (e.g., indinavir and atazanavir/ritonavir) and with other non-nucleoside reverse transcriptase inhibitors (e.g., etravirine).
Effects of Combined Hormonal Contraceptives on Other Drugs
Table 5 provides significant drug interaction information for drugs co-administered with TWIRLA.
Lamotrigine | |
Clinical effect |
|
Prevention or management | Dose adjustment may be necessary. Consult the approved product labeling for lamotrigine. |
Thyroid Hormone Replacement Therapy or Corticosteroid Replacement Therapy | |
Clinical effect | Concomitant use of CHCs with thyroid hormone replacement therapy or corticosteroid replacement therapy may increase systemic exposure of thyroid-binding and cortisol-binding globulin [see Warnings and Precautions (5.12 )] . |
Prevention or management | The dose of replacement thyroid hormone or cortisol therapy may need to be increased. Consult the approved product labeling for the therapy in use. [see Warnings and Precautions (5.12 )] . |
Other Drugs | |
Clinical effect | Concomitant use of CHCs may decrease systemic exposure of acetaminophen, morphine, salicylic acid, and temazepam. Concomitant use with ethinyl estradiol-containing CHCs may increase systemic exposure of other drugs (e.g., cyclosporine, prednisolone, theophylline, tizanidine, and voriconazole). |
Prevention or management | The dosage of drugs that can be affected by this interaction may need to be increased. Consult the approved product labeling for the concomitantly used drug. |
Effect on Laboratory Tests
The use of CHCs may influence the results of some laboratory tests, such as coagulation factors, lipids, glucose tolerance, and binding proteins.
Concomitant Use with HCV Combination Therapy – Liver Enzyme Elevation
CHCs are contraindicated for use with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Warnings and Precautions (5.4 ) and Contraindications (4 )] . Discontinue TWIRLA prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. TWIRLA can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.
DESCRIPTION
TWIRLA (levonorgestrel and ethinyl estradiol) transdermal system (TDS) contains 2.60 mg levonorgestrel (LNG) (17α)-(–) [13-ethyl-17¬hydroxy-18, 19-dinorpregn-4-en-20-yn-3-one], a progestin, and 2.30 mg ethinyl estradiol (EE), [(17α)-19-norpregna-1, 3, 5(10)-trien-20-yne-3, 17-diol] an estrogen (Figure 3).
Figure 3. Structural Formulas
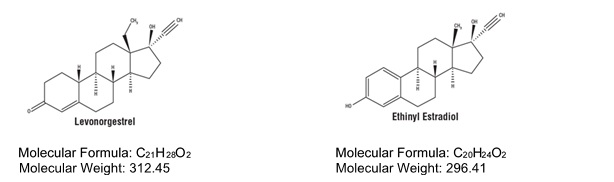
TWIRLA is designed to provide daily exposure of 120 mcg LNG and 30 mcg EE. TWIRLA is a matrix type TDS consisting of a 15 cm 2 active adhesive laminate center, surrounded by a peripheral inactive adhesive laminate. The entire area of TWIRLA is 28 cm 2 .
TWIRLA consists of 5 layers and a release liner which is removed and discarded prior to application. The two innermost layers contain the active ingredients (LNG and EE), as well as inactive components. Proceeding from the outer surface toward the surface adhering to the skin, the layers are (1) a woven peripheral backing layer, which is etched with “TWIRLA Levonorgestrel 120 mcg/day Ethinyl Estradiol 30 mcg/day”; (2) an inactive peripheral acrylic adhesive layer; (3) an inactive peripheral polyisobutylene adhesive layer; (4) an internal membrane to separate the active adhesive matrix from the inactive adhesive laminate; (5) the active adhesive matrix (Figure 4).
Figure 4. Schematic Depiction of the TWIRLA TDS
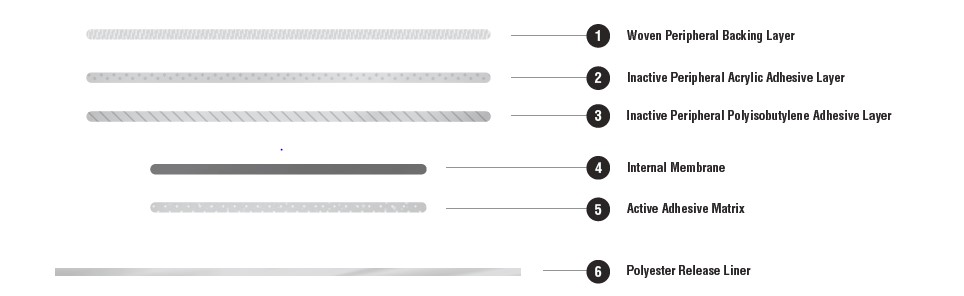
The inactive components are acrylic adhesives, capric acid, copovidone, crospovidone, dimethyl sulfoxide, ethyl lactate, lauryl lactate, polybutene, polyester internal membrane, polyester release liner, polyisobutylene adhesives, and woven polyester backing membrane. TWIRLA is not made with latex.
CLINICAL PHARMACOLOGY
Mechanism of Action
Combination hormonal contraceptives lower the risk of becoming pregnant primarily by suppressing ovulation.
Pharmacodynamics
TWIRLA exhibited ovulation inhibition as defined by serum progesterone concentrations. In one study subjects were treated with TWIRLA for three cycles. In this study, approximately 80% of these subjects had serum progesterone concentrations < 4.7 ng/mL.
Pharmacokinetics
TWIRLA is a TDS designed with an active matrix core containing LNG and EE. TWIRLA delivers medication to the systemic circulation by absorption of LNG and EE through the skin.
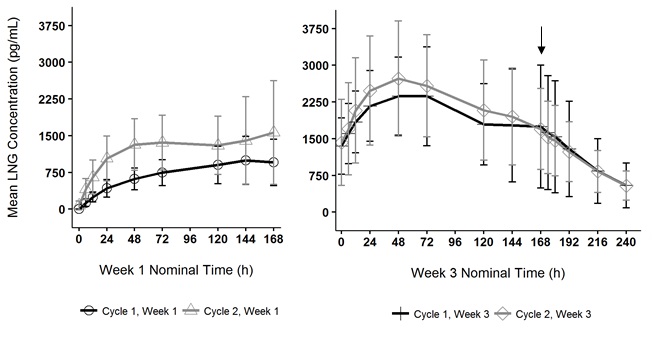
Absorption Following application of TWIRLA, both LNG and EE reach a plateau by 24 to 48 hours (Figures 5 and 6). Delivery of hormones is continuous over the 7 days of TWIRLA wear. The mean pharmacokinetic parameters (C ss and AUC 0‑168 ) for LNG and EE following two consecutive cycles of TWIRLA are summarized in Table 6.
NC: not calculable | |||||
| Analyte | Parameter | Cycle 1 Week 1 (N=18) | Cycle 1 Week 3 (N=18) | Cycle 2 Week 1 (N=18) | Cycle 2 Week 3 (N=18) |
| LNG | C ss (pg/mL) Average concentration within the 48-168 h time interval | 842 (41.2) | 2009 (47.2) | 1389 (46.5) | 2209 (44.5) |
| AUC 0-168 (ng∙h/mL) AUC0-168: area under the plasma drug concentration-time curve calculated between 0 and 168 h | 120.0 (39.1) | 339.0 (41.1) | 207.0 (44.1) | 378.0 (43.8) | |
| t 1/2 (h) t 1/2 : elimination half-life | NC | 38.2 (22.7) | NC | 40.5 (15.4) | |
| EE | C ss (pg/mL) | 31.9 (37.4) | 34.8 (37.4) | 38.6 (41.7) | 40.3 (38.9) |
| AUC 0-168 (pg∙h/mL) | 5040 (35.4) | 6210 (34.2) | 6060 (35.9) | 7120 (36.6) | |
| t 1/2 (h) | NC | 19.7 (18.8) | NC | 20.5 (18.2) | |
In multiple dose studies, AUC 0-168 for LNG and EE showed within-cycle and between cycle increases and the mean serum concentrations of EE and LNG were highest during the third Week of Cycle 2 after two consecutive cycles of wear (Figures 5 and 6). In a three-cycle study, the steady-state pharmacokinetics of EE and LNG was reached during Cycle 2. Upon removal of TWIRLA, serum levels of EE and LNG reach non‑measurable levels and low levels within 3 days, respectively.
Figure 5. Mean Serum Ethinyl Estradiol Concentrations in Healthy Female Volunteers Following Two Consecutive Cycles of TWIRLA Wear on the Buttock (Vertical arrow indicates time of TWIRLA removal)
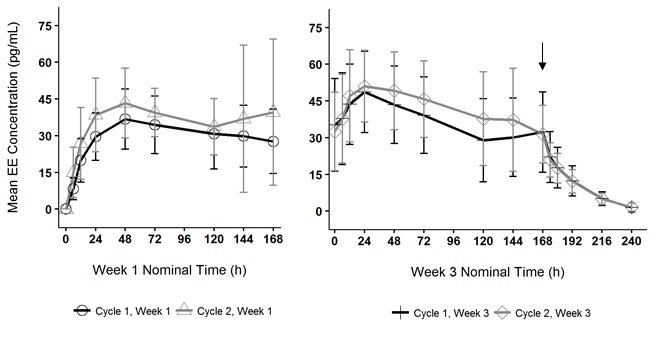
Figure 6. Mean Serum Levonorgestrel Concentrations in Healthy Female Volunteers Following Two Consecutive Cycles of TWIRLA Wear on the Buttock (Vertical arrow indicates time of TWIRLA removal)
The absorption of LNG and EE following application of TWIRLA to the buttock, abdomen, and upper torso (excluding the breasts) was examined. While absorption from the abdomen was slightly lower than from other sites, absorption from all three anatomic sites was considered to be therapeutically equivalent.
The absorption of LNG and EE following application of TWIRLA was studied under various external conditions including sauna, whirlpool, treadmill, and in a cold-water bath. Somewhat lower drug concentration levels were reported for whirlpool and treadmill with geometric ratios within the 78-90% range for both LNG and EE and dry sauna (LNG only).
Distribution LNG in serum is primarily bound to sex hormone-binding globulin (SHBG). EE is about 97% bound to plasma albumin. EE does not bind to SHBG but induces SHBG synthesis.
Elimination Metabolism Since TWIRLA is applied transdermally, first-pass metabolism (via the gastrointestinal tract and/or liver) of LNG and EE that would be expected with oral administration does not occur. Hepatic metabolism of LNG and EE occurs as described below.
Levonorgestrel: The most important metabolic pathways are reduction of the Δ4-3-oxo group and hydroxylation at positions 2α, 1β, and 16β, followed by conjugation. Most of the circulating metabolites are sulfates of 3α, 5β-tetrahydro-levonorgestrel, while excretion occurs predominantly in the form of glucuronides. Some of the parent LNG also circulates as 17β-sulfate. Metabolic clearance rates may differ among individuals by several-fold, and this may account in part for the wide variation observed in LNG concentrations among users.
Ethinyl estradiol: Cytochrome P450 enzymes (CYP3A4) in the liver are responsible for the 2-hydroxylation that is the major oxidative reaction. The 2-hydroxy metabolite is further transformed by methylation and glucuronidation prior to urinary and fecal excretion. Levels of CYP3A4 vary widely among individuals and can explain the variation in rates of EE 2-hydroxylation.
Excretion LNG and its metabolites are excreted in the urine (40% to 68%) and in feces (16% to 48%). The mean terminal elimination half-life for LNG in TWIRLA is approximately 41 ± 6.2 hours at steady state.
EE is excreted in the urine and feces as glucuronide and sulfate conjugates and undergoes enterohepatic recirculation. The terminal elimination half-life of EE in TWIRLA is approximately 21 ± 3.7 hours at steady state.
CLINICAL STUDIES
The efficacy of TWIRLA was evaluated in one open label, single arm, multicenter trial in the United States (Study 1) (NCT02158572) of one-year duration that enrolled 2,031 women, ranging in age between 18 and 60 years, who were healthy and sexually active with regular menstrual cycles. For the primary efficacy analysis, 1,736 women between the ages 18 and 35 years completed 15,165 evaluable 28-day cycles with TWIRLA, where no back-up contraception was used, and sexual intercourse occurred.
The racial/ethnic distribution for the primary analysis was White (67%), Black/African American (24%), Asian (4%), American Indian/Alaskan Native (0.5%), Native Hawaiian/Pacific Islander (0.5%), Other/Multiple races (5%); 19% of the study population were Hispanic. The mean age was 26 years.
The mean BMI in the primary efficacy analysis group was 28.3 kg/m 2 , and 35.3% of subjects had a BMI 30 kg/m 2 . The primary efficacy endpoint was the Pearl Index (PI) defined as the pregnancy rate per 100 woman-years of use. The overall PI for the primary analysis population (TWIRLA-treated patients) was 5.8 (95% CI 4.5, 7.2). There were clear differences in efficacy by BMI category as shown in Table 7 below.
| BMI | Number of evaluable cycles | Pearl Index (95% CI) |
| < 25 kg/m 2 | 6007 | 3.5 (1.8 - 5.2) |
| ≥ 25 and < 30 kg/m 2 | 3881 | 5.7 (3.0 - 8.4) |
| ≥ 30 kg/m 2 | 5264 | 8.6 (5.8 - 11.5) |
Figure 7 shows a model of the rate of pregnancy as BMI increases based on data from Study 1. There is an increase in pregnancy rate (i.e., the number of pregnancies per 100 woman-years), as BMI increased based on the primary analysis population (N = 1,735). TWIRLA is contraindicated in women with a BMI ≥ 30 kg/m 2 [see Indications and Usage (1 ) and Contraindications (4 )] .
Figure 7. Pregnancy Rates (Estimated•) in TWIRLA-Treated Patients as BMI Increases for Women ≤ 35 Years of Age in Study 1
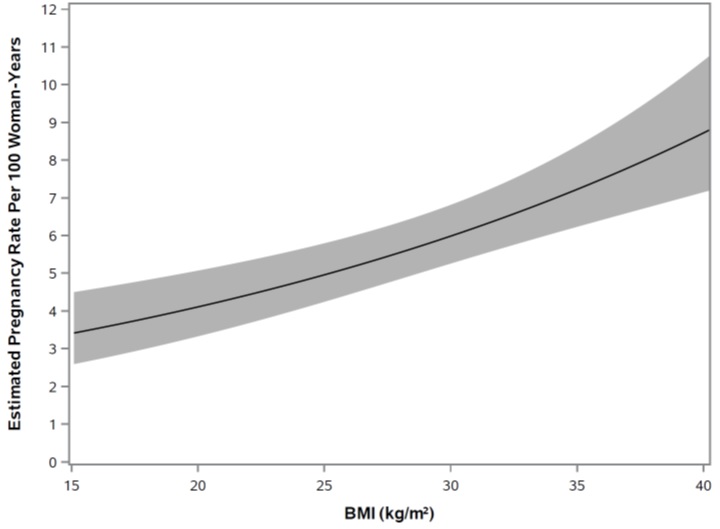
•Plot is based on Poisson Model with continuous BMI as the predictor (N=1,735); one woman in the primary analysis population had no BMI information. The solid line displays the estimated pregnancy rate, and the shaded area displays the 95% confidence interval for the estimated pregnancy rate.
Adhesion Based on a Phase 1 study in 78 subjects wearing one TWIRLA on the lower abdomen for 7 days, 77 systems applied (98.7%) exhibited 75% or greater surface area adhesion at all timepoints evaluated (every 24 hours) throughout the wear period. In the Phase 3 trial, 5.0% of all transdermal systems worn during the year-long trial (55,900 transdermal systems) fully detached. Subject-reported adhesion was generally better for the abdomen as compared to the upper torso and buttock. Full detachment rates were higher for transdermal systems exposed to water as compared to transdermal systems with no water exposure.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
TWIRLA (levonorgestrel and ethinyl estradiol) transdermal system is a beige 28 cm 2 round product etched with “TWIRLA Levonorgestrel 120 mcg/day Ethinyl Estradiol 30 mcg/day” and supplied as:
- a carton of 3 identical TDS, each TDS is packaged in an individual pouch. NDC 71671-100-03
- as a single TDS provided for replacement as needed. NDC 71671-100-01
Storage Conditions and Disposal
Store at room temperature 20°C to 25°C (68°F to 77°F) with excursions permitted 15°C to 30°C (59°F to 86°F) [ see USP Controlled Room Temperature ].
Store in original unopened pouch.
Used TDS still contain some active hormones. To discard, fold the sticky sides of the TDS together, place in a sturdy container, preferably with a child-resistant cap, and place this container in the trash. Used TDS should not be flushed down the toilet. See www.fda.gov/drugdisposal for more information about disposal of medicines.
INSTRUCTIONS FOR USE TWIRLA ® (TWER-la) (levonorgestrel and ethinyl estradiol) transdermal system |
TWIRLA is for skin use only.
Read this Instructions for Use before you start using the TWIRLA transdermal system (TDS) (also called a patch) and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your contraceptive treatment.
Do not cut, damage or change the TWIRLA patch in any way. If the patch is cut, damaged or changed in any way, it may be less effective.
Starting TWIRLA for the first time: If you are starting TWIRLA for the first time, you should wait until you begin your menstrual period.
- Day 1 Start . You should apply your first patch during the first 24 hours of your menstrual period. Your Patch Change Day will be on this day every week. If you start after Day 1 of your menstrual period, non-hormonal back up birth control (such as condoms and spermicide, or diaphragm and spermicide) should be used in addition to the patch for the first 7 days of your first patch cycle.
If you are changing from the oral hormone birth control pills, vaginal contraceptive ring or another transdermal patch to TWIRLA:
- Day 1 Start : You should apply your first patch during the first 24 hours of your menstrual period. Your Patch Change Day will be on this day every week. If you start after Day 1 of your menstrual period, non-hormonal back up birth control (such as condoms and spermicide, or diaphragm and spermicide) should be used in addition to the patch for the first 7 days of your first patch cycle.
- Finish your current oral hormone birth control pill cycle, vaginal ring cycle or other transdermal patch cycle. Apply your first TWIRLA patch on the day you would normally start your next oral birth control pill, patch or insert your next vaginal ring.
- If you do not get your period within 1 week after taking your last active oral hormone birth control pill, removing your last vaginal ring or other transdermal patch cycle, check with your healthcare provider to make sure you are not pregnant. You may still go ahead and start TWIRLA for contraception.
- If you apply your TWIRLA patch more than 1 week after taking your last active oral hormone birth control pill, removing your last vaginal ring or other transdermal patch cycle use a non-hormonal contraceptive method with the TWIRLA patch for the first 7 days of using the patch.
If you are starting TWIRLA after a miscarriage or abortion:
- You may start TWIRLA right away after a miscarriage or abortion that occurs in the first 12 weeks (first trimester) of pregnancy. If you start TWIRLA within 5 days of your first trimester abortion or miscarriage, you do not need to use another back up contraceptive method.
- If you do not start TWIRLA within 5 days after a first trimester miscarriage or abortion, use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, while you wait for your period to start.
- If you are starting TWIRLA after a miscarriage or abortion that occurs after the first 12 weeks of pregnancy (second trimester), wait 4 weeks before using TWIRLA and use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, for the first 7 days of your first patch cycle only.
If you are starting TWIRLA after childbirth:
- If you are not breastfeeding, wait 4 weeks before using TWIRLA and use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, for the first 7 days of your first patch cycle only. If you start using TWIRLA after childbirth and have not had your menstrual period, tell your healthcare provider. They will need to make sure you are not ovulating or pregnant before starting TWIRLA. If your healthcare provider tells you are not pregnant, use a non-hormonal contraceptive method for the first 7 days of patch of your first patch cycle.
How to Apply TWIRLA:
Where should the patch be applied?
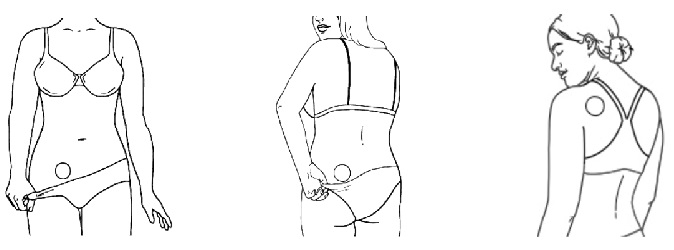
- Wear only 1 patch at a time.
- Before applying the patch, make sure your skin is clean and dry.
- Avoid using make up, creams, lotions, oils, powders or any other products on the skin area where you put or plan to put the patch.
- Application sites to apply the patch include the lower stomach area (abdomen), buttock, or the upper torso. See diagrams above.
- When you put the patch on, it should lay flat and smooth with no wrinkles or folds.
- On Patch Change Day , remove the current patch and immediately put on a new patch. Do not apply the new patch directly over skin where the old patch site was. You should use a new application site.
Where not to place the patch.
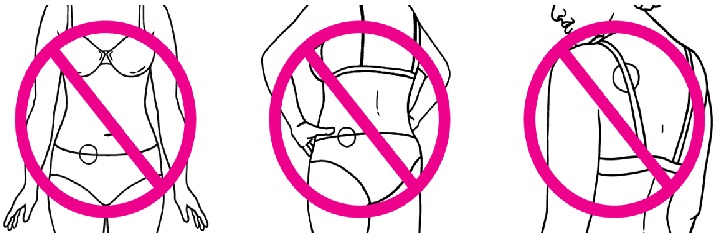
- Do not put the patch on your waistline or near clothing or undergarment seams.
- Do not put the patch on the breasts, on cut or irritated skin (rashes or other skin problems), or on the same location as the old patch.
 |
|
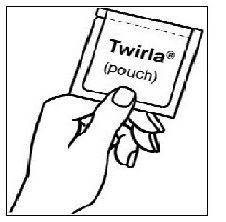
 | Step 1. Tear the pouch open at the notch on the pouch. |
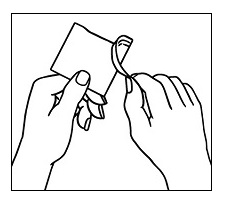
 | Step 2. Open the pouch and carefully remove the patch. The patch is attached to a clear protective liner. |
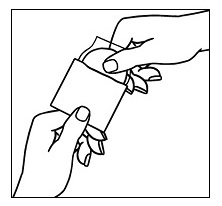
Avoid touching the sticky side of the patch 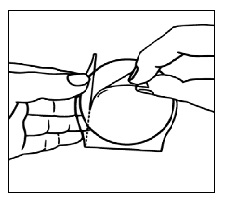 | Step 3a. Hold the patch with the clear protective liner facing you. You will see two sections: a large section and a small section. Avoid touching the sticky side of the patch with your fingers. Step 3b. Hold the small section of the liner. Remove and throw away (discard) the large section of the liner while still holding the small section of the liner. |
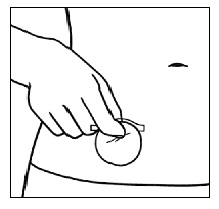 | Step 4. Hold the small section of the liner and apply the sticky side of the patch to the chosen patch site. |
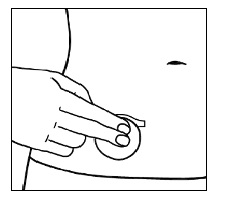 | Step 5. Press the sticky side of the patch firmly onto your skin and smooth it down. |
| Avoid wrinkles or folds | |
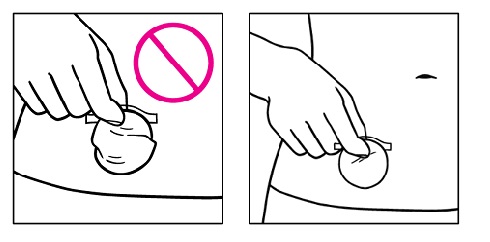 | Step 6. If the patch is not flat on the skin or there are large wrinkles, gently pull the patch off the skin while holding only the remaining protective liner and then put it on again. |
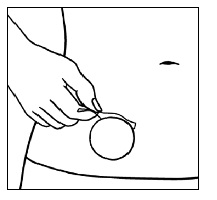 | Step 7. After the patch is flat with no wrinkles, pull an edge of the remaining protective liner and gently pull it off. |
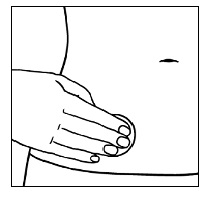 | Step 8a. After the patch is on your body, press the entire patch firmly into place with your hand for 10 seconds, making sure the edges stick well. Step 8b. Make sure the patch is on your skin all the way. |
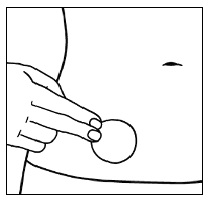 | Step 9. The edges of the patch should be smoothed over with your finger and make sure there is good contact around the patch with your skin and make sure there are no wrinkles. |
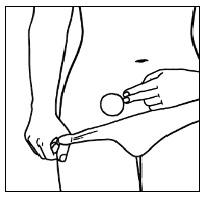 | Step 10. It is important that you check the patch every day to make sure it is in the right place. The patch should be checked after any water exposure (such as bathing, showering, or swimming) to make sure it is in the right place because water may affect how well the patch sticks to your skin . |
How do I throw away TWIRLA patches?
- To throw away your TWIRLA patch, fold the sticky side of the patch together and place in the trash right away so that children and pets cannot reach it. Do not flush used TWIRLA patches down the toilet.
- For more information on how to safely throw away medicines, see www.fda.gov/drugdisposal .
When should I change the TWIRLA patch?
- TWIRLA is applied in a 4 week patch cycle. Each patch cycle includes 4 weeks (28 days). You will put on 1 patch every week for 3 weeks. You will not wear a patch during week 4. Each patch is worn for 7 days (1 week).
- Apply a new TWIRLA patch on the same day each week (this is called your Patch Change Day). For example, if you apply your first patch on a Monday, all of your TWIRLA patches should be applied on Monday.
- You will not wear a TWIRLA patch during week 4 (this is called your Patch Free Week). Make sure you remove the old patch from your body. Your period should begin during your Patch Free Week.
- After you have finished week 4, apply a new TWIRLA patch on the day after Week 4 ends. Repeat the patch cycle of 1 patch a week for 3 weeks followed by your Patch Free Week.
What if a patch starts to lift off your skin or completely comes off?
- If your patch starts to lift off your skin or completely comes off and you do not replace it, you may not get enough hormones to keep you from getting pregnant.
- If a patch starts to lift off your skin or is completely off for less than one day (up to 24 hours), you should try and put it on again to the same place or replace it with a new patch immediately. No back up birth control is needed and your Patch Change Day will remain the same.
- If a patch starts to lift off your skin or is completely off for more than 1 day (24 hours or more) or if you are not sure how long the patch has been not attached to your skin, you may not be protected from pregnancy. You should stop your current patch cycle and start over on a new patch cycle right away by putting on a new patch. The day you apply your new patch is now your new Day 1 and your new Patch Change Day . Non-hormonal back up birth control, (such as condoms and spermicide, or diaphragm and spermicide) must be used for the first week of the new patch cycle.
- Do not put a patch on again if it is no longer sticky, if it has become stuck to itself or another surface or if it has other material stuck to it. If your patch cannot be put on again, a new patch should be put on right away. If you need help applying a patch, contact Agile Medical Information at 1-855-389-4752 or email: medicalaffairs@agiletherapeutics.com .
Can I wear the patch when I am exercising, or using a sauna, swimming pool, or whirlpool?
- Yes, women can maintain all their normal daily activities while using the patch.
- It is important to check your patch after any water that touches your patch during bathing, showering, or swimming, as prolonged water exposure may affect how well the patch sticks to your skin.
- If the patch starts to come off or completely lifts off the skin, try to put it on again.
- A patch should not be put on again if it is no longer sticky, if it has become stuck to itself or another surface or if it has other material stuck to it.
- If your current patch cannot be put on again, a new patch should be put on right away. Before applying the patch, make sure your skin is clean and dry.
- Make sure you have not used any make up, creams, lotions, oils, powders or any other products on the skin area where you put or plan to put the patch. If you find yourself in need of an additional patch because you needed to replace a patch, contact Agile Medical Information at 1-855-389-4752 or email: medicalaffairs@agiletherapeutics.com .
What if you forget to change your patch (left your patch on more than 7 days)?
- If you forget to change your patch at the start of any patch cycle (Day 1): You may not be protected from pregnancy. You should apply the first patch of your new patch cycle as soon as you remember. This is now your new Patch Change Day and your new Day 1. You must use non-hormonal back up birth control (such as condoms and spermicide, or diaphragm and spermicide) for the first week of your new patch cycle.
- If you forget to change your patch in the middle of the patch cycle (Day 8 or Day 15): for 1 or 2 days (up to 48 hours): you should apply a new patch right away. The next patch should be applied on your usual Patch Change Day . No back up birth control is needed.
- If you forget to change your patch for more than 2 days (48 hours or more): You may not be protected from pregnancy . You should stop your current patch cycle and start a new 4 week patch cycle right away by putting on a new patch. This is now your new Patch Change Day and your new Day 1 . You must use non-hormonal back up birth control for the first week of your new patch cycle.
Frequent Patch Situations | Will I have a New Patch- Change Day | Will I need to start a New 4 week Patch Cycle | Will I need a backup Birth Control method |
Did not apply patch on scheduled Day 1 of new patch cycle | Yes | Yes | Yes (for 7 days) |
Patch not attached for less than 24 hours | No | No | No |
Patch not attached for 24 hours or more, or unsure how long | Yes | Yes | Yes (for 7 days) |
Less than 48 hours late for Patch Change Day (Day 8 or 15) | No | No | No |
48 hours or more late for Patch Change Day (Day 8 or 15) | Yes | Yes | Yes (for 7 days) |
Forgets to remove last patch on Day 22 | No | No | No |
What if you forget to remove your patch for the patch free week?
- Past Day 22 : You should take it off as soon as you remember. No other change is needed. You should still start the next patch cycle on the usual Patch Change Day , which is the day after Day 28. No back up birth control is needed.
- Your TWIRLA patch should never be off more than 7 days in a row. If you ever go more than 7 days without a patch, you should use another birth control method.
- As with all hormonal birth control, the risk of getting pregnant increases with each day you go past the recommended 7 day patch free period.
What if you wish to change your Patch Change Day?
- If you want to change your Patch Change Day you should complete your current patch cycle, removing the third patch on the correct day. During the Patch Free Week, you may select an earlier Patch Change Day by applying a new patch on the chosen day.
Your TWIRLA patch should never be off more than 7 days in a row.
Manufactured by: Corium International, Inc. 4558 50th Street, SE Grand Rapids, MI 49512
Manufactured for: Agile Therapeutics, Inc. 500 College Rd. E, Suite 310 Princeton, NJ 08540
For more information call 1-855-389-4752 or email: medicalaffairs@agiletherapeutics.com .
This Instructions for Use has been approved by the U.S. Food and Drug Administration | Approved: 04/2022 |
Mechanism of Action
Combination hormonal contraceptives lower the risk of becoming pregnant primarily by suppressing ovulation.