Get your patient on Varithena (Polidocanol)
Varithena prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Varithena patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
For intravenous use only.
VARITHENA is intended for intravenous injection using ultrasound guidance, administered via a single cannula into the lumen of the target incompetent trunk veins or by direct injection into varicosities. Use up to 5 mL per injection and no more than 15 mL per session.
Physicians administering VARITHENA must be experienced with venous procedures and be trained in the administration of VARITHENA.
Activate VARITHENA using the VARITHENA oxygen canister and polidocanol canister ( see Instructions for Use ). Once a VARITHENA transfer unit is in place, foam can be generated and transferred to a syringe. Discard the syringe contents if there are any visible bubbles. Administer the injectable foam within 75 seconds of extraction from the canister to maintain injectable foam properties. Use a new sterile syringe after each injection. Use a new VARITHENA transfer unit for each treatment session.
Local anesthetic may be administered prior to cannula insertion but neither tumescent anesthesia nor patient sedation is required. Cannulate the vein to be treated using ultrasound guidance to confirm venous access.
Inject freshly generated VARITHENA injectable foam slowly (approximately 1 mL/second in the GSV and 0.5 mL/second in accessory veins or varicosities) while monitoring using ultrasound. Confirm venospasm of the treated vein using ultrasound.
When treating the proximal GSV, stop the injection when VARITHENA is 3-5 cm distal to the saphenofemoral junction (SFJ).
Apply compression bandaging and stockings and have the patient walk for at least 10 minutes, while being monitored. Maintain compression for 2 weeks after treatment.
Repeat treatment may be necessary if the size and extent of the veins to be treated require more than 15 mL of VARITHENA. Separate treatment sessions by a minimum of 5 days.
Retained coagulum may be removed by aspiration (microthrombectomy) to improve comfort and reduce skin staining.

By using PrescriberAI, you agree to the AI Terms of Use.
Varithena prescribing information
INDICATIONS AND USAGE
VARITHENA (polidocanol injectable foam) is indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins, and visible varicosities of the great saphenous vein (GSV) system above and below the knee. VARITHENA improves the symptoms of superficial venous incompetence and the appearance of visible varicosities.
DOSAGE AND ADMINISTRATION
For intravenous use only.
VARITHENA is intended for intravenous injection using ultrasound guidance, administered via a single cannula into the lumen of the target incompetent trunk veins or by direct injection into varicosities. Use up to 5 mL per injection and no more than 15 mL per session.
Physicians administering VARITHENA must be experienced with venous procedures and be trained in the administration of VARITHENA.
Activate VARITHENA using the VARITHENA oxygen canister and polidocanol canister ( see Instructions for Use ). Once a VARITHENA transfer unit is in place, foam can be generated and transferred to a syringe. Discard the syringe contents if there are any visible bubbles. Administer the injectable foam within 75 seconds of extraction from the canister to maintain injectable foam properties. Use a new sterile syringe after each injection. Use a new VARITHENA transfer unit for each treatment session.
Local anesthetic may be administered prior to cannula insertion but neither tumescent anesthesia nor patient sedation is required. Cannulate the vein to be treated using ultrasound guidance to confirm venous access.
Inject freshly generated VARITHENA injectable foam slowly (approximately 1 mL/second in the GSV and 0.5 mL/second in accessory veins or varicosities) while monitoring using ultrasound. Confirm venospasm of the treated vein using ultrasound.
When treating the proximal GSV, stop the injection when VARITHENA is 3-5 cm distal to the saphenofemoral junction (SFJ).
Apply compression bandaging and stockings and have the patient walk for at least 10 minutes, while being monitored. Maintain compression for 2 weeks after treatment.
Repeat treatment may be necessary if the size and extent of the veins to be treated require more than 15 mL of VARITHENA. Separate treatment sessions by a minimum of 5 days.
Retained coagulum may be removed by aspiration (microthrombectomy) to improve comfort and reduce skin staining.
DOSAGE FORMS AND STRENGTHS
VARITHENA is available in the following presentations:
- 180 mg/18 mL (10 mg/mL)
- 77.5 mg/7.75 mL (10 mg/mL)
Once activated, VARITHENA is a white, injectable foam delivering a 1% polidocanol solution.
Each mL of VARITHENA injectable foam contains 1.3 mg of polidocanol
USE IN SPECIFIC POPULATIONS
Pregnancy
Few published case reports with use of polidocanol-containing products, including VARITHENA, in pregnant women have not identified any drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes. Although no risks have been identified, there is minimal benefit in treating lower extremity varicosities during pregnancy and lower extremity varicosities that develop during pregnancy as they may spontaneously regress postpartum. In animal reproduction studies, no adverse developmental effects were observed with intravenous administration of polidocanol to pregnant rats and rabbits during organogenesis at dose levels up to approximately 13.5 and 12 times, respectively, the proposed maximum human dose of 15 mL of 1% VARITHENA based on body surface area (see Data ) .
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data Animal Data
Developmental reproductive toxicity testing was performed in rats and rabbits using intravenous administration of polidocanol solution. In rabbits, dose levels up to and including 10 mg/kg/day (approximately 12 times the proposed maximum human dose of 15 mL of 1% VARITHENA based on body surface area) did not produce any indication of adverse effects on embryo-fetal mortality, fetal weight, or the incidences of fetal abnormalities and variants. In rats administered 27 mg/kg/day of polidocanol solution (approximately 13.5 times the human dose based on body surface area), there were no adverse effects on pregnancy performance or fetal development. In a peri-natal and post-natal study in rats, dose levels of polidocanol up to 9 mg/kg/day (approximately 4.5 times the human dose based on body surface area) were without effects on the development of the conceptus and offspring, and at a dose level of 27 mg/kg/day of polidocanol solution (approximately 13.5 times the human dose based on body surface area), effects were confined to an equivocal reduction in body weights of first-generation males, and an associated equivocal delay in the age of preputial separation.
Lactation
There are no data on the presence of polidocanol in human milk, the effects on the breastfed infant, or the effects on milk production. A lactating woman may consider interrupting breastfeeding and pumping anddiscarding breast milk up to 8 hours after VARITHENA administration in order to minimize exposure to a breastfed infant.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Of the 1333 subjects in clinical studies treated with VARITHENA, 9.1% (n=121) were ≥65 years of age. No clinically important differences in safety or efficacy were observed between older and younger patients in all studies.
CONTRAINDICATIONS
The use of VARITHENA is contraindicated in patients with:
- known allergy to polidocanol [see Warnings and Precautions (5.1 )]
- acute thromboembolic disease
WARNINGS AND PRECAUTIONS
Anaphylaxis
Severe allergic reactions have been reported following administration of liquid polidocanol, including anaphylactic reactions, some of them fatal. Observe patients for at least 10 minutes following injection and be prepared to treat anaphylaxis appropriately.
Tissue Ischemia and Necrosis
Intra-arterial injection or extravasation of polidocanol can cause severe necrosis, ischemia or gangrene Patients with underlying arterial disease, such as marked peripheral arteriosclerosis or thromboangiitis obliterans (Buerger’s Disease) may be at increased risk for tissue ischemia. If intra-arterial injection of polidocanol occurs, consult a vascular surgeon immediately.
Venous Thrombosis
VARITHENA can cause venous thrombosis [see Adverse Reactions (6 )] . Follow administration instructions closely and monitor for signs of venous thrombosis after treatment. Patients with reduced mobility, history of deep vein thrombosis or pulmonary embolism, or recent (within 3 months) major surgery, prolonged hospitalization, or pregnancy are at increased risk for developing thrombosis.
ADVERSE REACTIONS
In clinical trials, the most common related adverse events (occurring in ≥3% of patients treated with VARITHENA) were pain/discomfort in extremity, infusion site thrombosis (retained coagulum), injection site hematoma or pain, thrombophlebitis superficial, and extravasation.(6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Biocompatibles, Inc. at 1-855-971-VEIN (1-855-971-8346) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Clinical Trials Experience
Because clinical trials are conducted under controlled but widely varying conditions, adverse reaction rates observed in clinical trials of VARITHENA cannot be directly compared to rates in the clinical trials of other drugs or procedures and may not reflect the rates observed in practice.
A total of 1333 patients with GSVI in 12 clinical trials were evaluated for safety when treated with VARITHENA at dose concentrations of 0.125%, 0.5%, 1.0%, or 2.0%, including 437 patients treated with VARITHENA in placebo-controlled clinical trials.
Adverse reactions occurring in 3% more patients receiving VARITHENA 1% than receiving placebo are shown in Table 1 .
| a Retained coagulum. b Common femoral vein thrombus extension (non-occlusive thrombi starting in the superficial vein and extending into the common femoral vein). | ||
| Table 1: Treatment-emergent adverse reactions (3% more on VARITHENA 1% than on placebo) through Week 8 (n=588) | ||
| Adverse Reaction | Placebo (N=151) | VARITHENA 1.0% (N=149) |
| Pain in extremity | 14 (9.3) | 25 (16.8) |
| Infusion site thrombosis b | 0 | 24 (16.1) |
| Contusion/injection site hematoma | 9 (6.0) | 23 (15.4) |
| Limb discomfort | 5 (3.3) | 18 (12.1) |
| Tenderness/injection site pain | 5 (3.3) | 16 (10.7) |
| Venous thrombosis limb c | 0 | 12 (8.1) |
| Thrombophlebitis superficial | 2 (1.3) | 8 (5.4) |
| Deep vein thrombosis | 0 | 7 (4.7) |
In VARITHENA-treated patients, 80% of pain events in the treated extremity resolved within 1 week.
Proximal symptomatic venous thrombi occurred in <1% of patients treated with VARITHENA. Approximately half of patients with thrombi received treatment with anticoagulants.
Since VARITHENA induces thrombosis in the treated superficial veins, D-dimer is commonly elevated post-treatment and is not useful diagnostically to assess patients for venous thrombus following treatment with VARITHENA.
Neurologic adverse events (cerebrovascular accident, migraines) have been reported in patients following administration of physician compounded foam sclerosants. None of the 1333 patients in the VARITHENA trials experienced clinically important neurological or visual adverse events suggestive of cerebral gas embolism. The incidence of neurologic and visual adverse events within 1 day of treatment in the placebo-controlled studies was 2.7% in the pooled VARITHENA group and 4.0% in the placebo groups.
Skin discoloration adverse events were reported in 1.1% of the pooled VARITHENA group and 0.7% of the placebo group in the placebo-controlled studies.
DRUG INTERACTIONS
No specific drug interaction studies have been performed. There are no known drug interactions with VARITHENA.
DESCRIPTION
VARITHENA injectable foam contains the sclerosant, polidocanol. It is intended for intravenous use only.
Chemically, polidocanol is polyoxyl lauryl ether. The structural formula is represented below:
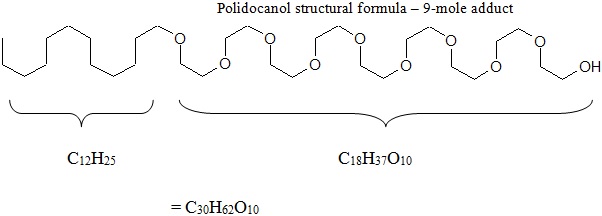
Polidocanol has the molecular formula CH 3 (CH 2 ) 11 (OCH 2 CH 2 ) n OH and a molecular weight of 582.9 when the average ethylene glycol moieties is nine (n=9). Polidocanol is a white to almost white, waxy, hygroscopic solid that is soluble in water and alcohol and melts at temperatures above 20°C.
VARITHENA is a sterile, injectable foam of an aqueous polidocanol solution (1%) containing the following inactive ingredients: alcohol (4.2% w/w, purity 96% v/v) disodium hydrogen phosphate dihydrate (0.24% w/w), and potassium dihydrogen phosphate (0.085% w/w) with a pH of 6.0-7.5.
The injectable foam is generated after activation of the polidocanol canister with oxygen from a second aluminum canister, resulting in a final gas mixture of oxygen:carbon dioxide in a ratio of 65:35 with low (<0.8%) nitrogen content. At the time of use, VARITHENA is generated as an injectable foam of controlled density and bubble size. The foam is then transferred to a syringe through the VARITHENA transfer unit. The injectable foam has a liquid to gas ratio of approximately 1:7 by volume. The median bubble diameter is less than 100 µm and no bubbles are greater than 500 µm.
CLINICAL PHARMACOLOGY
Mechanism of Action
VARITHENA is a drug/device combination product that generates injectable foam. The injectable foam is composed of a liquid and gas phase, both of which are necessary to have its therapeutic effect. VARITHENA is intended to act as follows: (1) the foam displaces blood from the vein to be treated, and (2) the polidocanol then scleroses the endothelium.
The active pharmaceutical ingredient of VARITHENA is polidocanol, a non-ionic surfactant sclerosing agent. The hydrophobic pole of the polidocanol molecule attaches to the lipid cell membrane of the venous endothelium, resulting in disruption of the osmotic barrier, destruction of the venous endothelium, and vasospasm. Following exposure to polidocanol, the interior surface of the vein becomes thrombogenic, which leads to thrombus formation and venous occlusion. The occluded vein is eventually replaced by fibrous connective tissue. Polidocanol is deactivated upon contact with blood, thus limiting the sclerosant action to the endothelium near the site of injection.
Pharmacodynamics
The active pharmaceutical ingredient in VARITHENA is polidocanol. Polidocanol damages the endothelium of blood vessels.
Pharmacokinetics
The pharmacokinetics of VARITHENA (as a weighted sum of 4 oligomers: E5, E9, E12 and E14) were evaluated at two concentrations (1% and 2%) randomly assigned within gender in 20 patients with GSV incompetence.
When administered as an intravenous injectable foam as two fixed 5 mL doses separated by 10 minutes, polidocanol was rapidly detected in plasma, reaching maximum concentration of drug in the body after dosing (C max ) within 15 minutes of the first injection and within 5 minutes of receiving the second injection of VARITHENA 1% or VARITHENA 2%. The mean volume of distribution (Vd) of polidocanol ranged from 35 to 82 L.
Mean systemic clearance (CL) of polidocanol ranged from 0.2 to 0.4 L/min. The clearance of E5 was significantly greater than that of longer oligomers. Mean terminal elimination half-life (t 1/2 ) ranged from 102 to 153 minutes, with most plasma samples below the limit of quantitation (BLQ) at the end of the 8-hour collection period. The increase in plasma polidocanol concentrations was less than proportional with increasing VARITHENA concentration. Weight-normalized data demonstrated no consistent differences in C max or AUC between males and females.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential of VARITHENA. No mutagenic activity was observed in the in vitro bacterial reverse mutation assay at non-toxic concentrations. No mutagenic activity was observed in the in vitro mouse lymphoma assay in the absence of S9 mix and was weakly mutagenic in the presence of S9 close to the limit of acceptance for the accompanying level of toxicity. No micronucleus induction was detected in the in vivo assay on mouse bone marrow cells up to the maximum tolerated dose of 80 mg/kg.
There was no adverse effect on fertility in both male and female rats at 27 mg/kg/day. This dose level is approximately 13.5 times the proposed maximum human dose based on body surface area.
Animal Toxicology and/or Pharmacology
The pharmacological effects of polidocanol solution on the renal function of the rat were evaluated and at the highest dose tested (10 mg/kg) hematuria occurred in 67% of animals. This dose is 5 times higher than the proposed maximum human dose based on body surface area. Blood was no longer detectable in urine 24 hours after dosing. In the 28-day repeated dose toxicity study in rat blood, pigments were noted in the urine for animals in all treatment groups, including male controls, at the end of the 4-week treatment period with up to 27 mg polidocanol/kg/day. Following the 2-week recovery period, there was still evidence that blood pigments were present in the urine but the incidence and severity was decreased when compared to the main study animals. There were no histopathological findings in the urinary bladder in any study animals.
In a cardiovascular pharmacology study in the anesthetized dog at 20 mg/kg (approximately 33 times the human dose based on body surface area), statistically significantly higher values for P-Q interval were measured before and during dosing and at all time points up to 30 minutes after dosing. An increase in QRS interval was also measured after dosing of 20 mg/kg and at 5 and 10 minutes after dosing. This effect was short lived and was no longer seen at 15 minutes after dosing. In addition, there was an increase in diastolic pressure with increasing dose of polidocanol. This increase became significantly greater (p<0.05) than baseline before injection of the final and highest dose (20 mg/kg).
In a further cardiovascular pharmacology study conducted with a once weekly, for four weeks, intravenous bolus injection of VARITHENA in the conscious dog, dose levels of up to 8.0 mL/kg (approximately 17 times the human dose based on body surface area) to beagle dogs caused only a transient, but consistent, effect on respiration, evidenced by a decrease in tidal volume and RMV at 15 minutes post-dose, resolving by one hour post-dose. Histopathology of the lung at the end of the 3-month follow-up period showed no abnormalities.
CLINICAL STUDIES
VARITHENA was evaluated in two randomized, blinded, multicenter clinical trials designed to assess the efficacy and safety of VARITHENA 0.5%, 1.0%, and 2.0% (VANISH-1) and VARITHENA 0.5% and 1.0% (VANISH-2) compared with placebo in the treatment of both symptoms and appearance in patients with SFJ incompetence as evidenced by reflux of the GSV or major accessory veins. In both studies, a VARITHENA 0.125% treatment group was included as a control for blinding of the duplex ultrasound assessment. Patients with history of deep vein thrombosis or pulmonary embolism; inability to comply with post-treatment compression due to severe peripheral arterial disease or leg obesity; incompetence of the small saphenous vein or deep venous reflux as a major source of reflux; or reduced mobility, major surgery, pregnancy, or prolonged hospitalization within 3 months were excluded. Patients were randomized in an equal distribution to each treatment group; the primary time point for analyses of the primary, secondary, and tertiary efficacy endpoints was Week 8.
In these clinical trials, the maximum volume of injectable foam or placebo to be administered per treatment session was 15 mL.
In VANISH-1, patients received one blinded treatment and in VANISH-2, patients received one blinded treatment with an option for a second blinded treatment 1 week later. In VANISH-2, patients in the VARITHENA 1.0% treatment group received an average of 1.4 blinded treatments. All patients received post-procedure compression therapy for 14 days following treatment.
Of the 519 patients randomized into VANISH-1 and VANISH-2, a total of 511 were treated with either VARITHENA 0.5% (n=111), 1.0% (n=110), or 2.0% (n=63), VARITHENA 0.125% as control (n=114), or placebo (n=113). Ninety-nine percent of the patients in VANISH-1 and VANISH-2 completed the blinded treatment period.
In the VARITHENA 1% group in VANISH-2, 23 of 58 patients received an additional blinded treatment. Two of these patients had retreatment of veins treated in the initial treatment session. The remaining 21 patients received treatment for additional veins not treated in the initial treatment session.
The mean age was approximately 50 years and approximately three-fourths of the patients were women. The mean BMI was similar in VANISH-1 and VANISH-2, at 28 kg/m 2 (range 16 to 44 kg/m 2 ) and 30 kg/m 2 (range 17 to 48 kg/m 2 ), respectively. The mean baseline GSV diameter was also similar in VANISH-1 and VANISH-2, at 7.6 mm (range 1.5 to 25.9 mm) and 8.7 mm (range 3.1 to 19.4 mm), respectively. Overall, 22% of patients in VANISH-1 and 25% of patients in VANISH-2 reported one or more prior varicose vein procedures in the leg to be treated.
For both clinical trials, the primary efficacy endpoint was improvement in patient symptoms, as measured by the change from baseline to Week 8 in the 7-day average electronic daily diary VVSymQ ® score. The VVSymQ ® score is a patient-reported outcome measure based on daily patient assessment of the varicose vein symptoms determined to be most important to patients: heaviness, achiness, swelling, throbbing, and itching. VVSymQ ® scores range from 0 to 25, where 0 represents no symptoms and 25 represents all 5 symptoms experienced all of the time. Results are shown in Table 2 .
For both VANISH-1 and VANISH-2, treatment with 1.0% was superior to placebo in improving symptoms as measured by VVSymQ ® , when either a duration or an intensity scale was used to measure patients’ symptoms.
| •Percent of patients who reported their symptoms had "moderately improved" or "much improved" compared with baseline. | ||||||
| Table 2: Improvement in Symptoms of Varicose Veins as Measured by VVSymQ ® at Week 8, VANISH-1 and VANISH-2 | ||||||
| VVSymQ ® | ||||||
| VANISH-1 | VANISH-2 | |||||
| Placebo | VARITHENA 1.0% | Placebo | VARITHENA 1.0% | |||
| N | 55 | 50 | 54 | 57 | ||
| Baseline score, mean | 8.60 | 8.82 | 9.26 | 7.82 | ||
| Adjusted mean change from baseline at week 8 | -2.13 | -4.87 | -2.00 | -5.06 | ||
| Clinically meaningful improvement in symptoms at week 8 • | 5.4% (n=56) | 64.7% (n=51) | 19.6% (n=56) | 75.9% (n=58) | ||
| Comparison vs. Placebo at week 8, p -value, adjusted mean change | <0.0001 | <0.0001 | ||||
The co-secondary endpoints in VANISH-1 and VANISH-2 were the improvement in appearance of visible varicosities from baseline to Week 8 as measured by 1) patients scoring the appearance of their varicose veins in the medial view of their study leg (PA-V 3 score) from "Not at all noticeable" (a score of 0) to "Extremely noticeable" (a score of 4); and 2) an independent photography review panel rating the severity of the patient's varicose vein appearance in standardized digital photographs of the medial view of each patient's study leg (IPR-V 3 score) from "None" (a score of 0) to "Very severe" (a score of 4). Results are shown in Table 3 .
| † Percent who reported the appearance of varicose veins had “moderately improved” or “much improved” compared with baseline. | ||||
| Table 3: Improvement in Appearance of Visible Varicosities as Measured by IPR-V 3 and PA-V 3 at Week 8, VANISH-1 and VANISH-2 | ||||
| VANISH-1 | VANISH-2 | |||
| Placebo | VARITHENA 1.0% | Placebo | VARITHENA 1.0% | |
| IPR-V 3 | ||||
| n | 55 | 49 | 56 | 57 |
| Baseline score, mean | 1.82 | 1.98 | 2.18 | 2.02 |
| Adjusted mean change from baseline at week 8 | -0.01 | -0.76 | -0.07 | -0.83 |
| Clinically meaningful improvement in appearance at week 8 † | 8.9% (n=56) | 70.6% (n=51) | 0 (n=56) | 70.7% (n=58) |
| Comparison vs. Placebo, p -value at week 8, adjusted mean change | <0.0001 | <0.0001 | ||
| PA-V 3 | ||||
| N | 55 | 50 | 56 | 57 |
| Baseline score, mean | 3.49 | 3.46 | 3.30 | 3.49 |
| Adjusted mean change from baseline at week 8 | -0.15 | -1.60 | -0.32 | -1.79 |
| Clinically meaningful improvement in appearance at week 8 † | 3.6% (n=56) | 54.9% (n=51) | 7.1% (n=56) | 69.0% (n=58) |
| Comparison vs. Placebo, p -value at week 8, adjusted mean change | <0.0001 | <0.0001 | ||
Tertiary endpoints in VANISH-1 and VANISH-2 included response to treatment as determined by change from baseline in Venous Clinical Severity Score (VCSS), by duplex ultrasound, and by change from baseline in Venous Insufficiency Epidemiologic and Economic Study – Quality of Life/Symptoms (VEINES-QOL) score.
VCSS is a clinician rating of severity of chronic venous insufficiency ranging from 0 to 30, where higher scores indicate more severe venous disease. In VANISH-1 and VANISH-2, the adjusted mean changes from baseline in VCSS in the 1% VARITHENA treatment groups were 3.70 and 5.05, respectively, at Week 8 compared with 0.75 and 1.52 points in the placebo groups, respectively. For both studies, the differences between these improvements are statistically significant ( P <0.0001).
The physiological response to treatment as measured by duplex ultrasound (duplex response) was defined as elimination of reflux through the SFJ and/or complete occlusion of all incompetent GSV and major accessory veins at baseline. The primary comparison for duplex response in both studies was the pooled VARITHENA groups versus the VARITHENA 0.125% (control) group. Results are shown in Table 4 .
| •In VANISH-1, a significant dose-response trend was evident between the percent of responders and the dose concentration of VARITHENA ( P< 0.0001). | |||
| Table 4: Response to Treatment as Measured by Duplex Ultrasound at Week 8, VANISH-1 and VANISH-2 | |||
| Parameter | Treatment Group, % | ||
| Placebo | VARITHENA 0.125% (control) | VARITHENA 1.0% | |
| Responders, VANISH-1 • | 5.4% (n=56) | 42.1% (n=57) | 80.4% (n=51) |
| Responders, VANISH-2 | 1.8% (n=56) | 59.6% (n=57) | 86.2% (n=58) |
VEINES-QOL is a disease-specific quality of life instrument, ranging from 0 (worst possible quality of life) to 100 (best possible quality of life). In VANISH-1 and VANISH-2, the adjusted mean changes from baseline in VEINES-QOL in the pooled VARITHENA treatment groups were 21.2 and 21.6, respectively, at Week 8 compared with 7.7 and 7.4 points in the placebo groups, respectively. For both studies, the differences between these improvements are statistically significant ( P< 0.0001).
For efficacy endpoints, VARITHENA treatment effects were consistent across subgroups of age, sex, BMI (up to 48 kg/m 2 ), CEAP clinical class, GSV diameter (up to 25.9 mm), and VCSS.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
VARITHENA (polidocanol injectable foam) product is available in four configurations, each containing two sterile, connected, 303-mL aluminum alloy cylinders, one containing polidocanol solution (10 mg/mL) under carbon dioxide, and the other containing pressurized oxygen.
| •Ancillary Pack includes three 10-mL syringes, one 20-inch manometer tube, and two compression pads. | ||||
| Polidocanol mg | Usable foam mL | Administration Pack | NDC | |
| Transfer units | Ancillary Pack• | |||
| 77.5 | 15 | 0 | 0 | 60635-107-01 PD Canister - 60635-007-01 |
| 1 | 1 | 60635-111-01 PD Canister - 60635-007-01 | ||
| 180 | 45 | 0 | 0 | 60635-118-01 PD Canister - 60635-018-01 |
| 3 | 3 | 60635-133-01 PD Canister - 60635-018-01 | ||
Storage and Handling
Do not shake VARITHENA canisters.
Avoid contact with eyes.
Store the VARITHENA Bi-Canister or convenience box at or below 86°F (30°C);
Do not refrigerate or freeze.
Unused, non-activated VARITHENA canisters may be stored in the flat or upright position.
Contains gas under pressure: May explode if heated. Store in a well-ventilated place. Store the canisters away from sources of heat including strong light conditions.
Pressurized Oxygen: May cause or intensify fire; oxidizer. Store away from combustible materials.
Once activated, the canister of 180 mg/18 mL (10 mg/mL) VARITHENA must be used within thirty (30) days.
Once activated, the canister of 77.5mg/7.75mL (10mg/mL) VARITHENA must be used within thirty (30) days.
Store activated canisters of VARITHENA upright, with the VARITHENA transfer unit attached, under the same temperature conditions as the VARITHENA Bi-Canister or convenience box. Use a new VARITHENA transfer unit for each treatment session.
Discard aerosol canisters after use in accordance with state and local requirements.
For more information, please refer to the IFU.
INSTRUCTIONS FOR USE
VARITHENA Delivery System
Instructions for Use
Please read all prescribing information before using the product.
Rx Only
The Instructions for Use are for the entire VARITHENA system. There are 4 packaging configurations:
180 mg/18 mL Configuration:
Option A: Bi-Canister box and Administration Pack•
Option B: Convenience box (Bi-Canister Box + 3 Ancillary Packs + 3 VARITHENA transfer units)•
•The components in each packaging configuration are to be used only in conjunction with each other for activation of VARITHENA. Administration Packs can be used for either configuration for further treatment sessions.
180 mg/18 mL Configuration: Always write the activation date and time on the canister and verify the product has not expired prior to use. Once the VARITHENA canister has been activated, the shelf life for the product is thirty (30) days.
A canister of VARITHENA generates 90 mL of foam which, following purging instructions contained in this IFU, is sufficient to yield 45 mL of usable foam for injection. The gas mix of the foam is 65:35 O 2 : CO 2 .
77.5 mg/7.75 mL Configuration:
Option A: Bi-Canister box and Administration Pack•
Option B: Convenience box (Bi-Canister Box + 1 Ancillary Pack + 1 VARITHENA transfer unit)•
•The components in each packaging configuration are to be used only in conjunction with each other for activation of VARITHENA.
77.5 mg/7.75 mL Configuration:Always write the activation date and time on the canister and verify the product has not expired prior to use. Once the VARITHENA canister has been activated, the shelf life for the product is thirty (30) days.
A canister of VARITHENA generates 30 mL of foam which, following purging instructions contained in this IFU, is sufficient to yield 15 mL of usable foam for injection. The gas mix of the foam is 65:35 O 2 : CO 2 .
WARNINGS:
As the foam fills the syringe and before injecting, inspect the syringe full of foam for any visible bubbles. If there are any present, the foam should be emptied into the VARITHENA transfer unit waste chamber and the syringe refilled.
Do not shake VARITHENA canisters.
Always use a fresh pair of sterile gloves when handling the Bi-Canister and VARITHENA transfer unit.
Notes: Use a new sterile syringe after each injection. Never fill a syringe until just before the foam is required.
Use foam within 75 seconds of generation or discard and generate new foam.
The activated VARITHENA canister should always be stored with a VARITHENA transfer unit in place in the upright position at or below 86°F (30°C) - do not refrigerate or freeze, in an appropriately controlled clean area to limit contamination. A new VARITHENA transfer unit must be used for each treatment session.
Unpacking VARITHENA:
Option A for 180 mg/18 mL: Bi-Canister Box and Administration Pack
Option A for 77.5 mg/7.75 mL: Bi-Canister Box and Administration Pack
Gather all the items needed for the generation of foam: the VARITHENA Bi-Canister box (Figure 1a ) , Administration Pack (including: VARITHENA transfer unit, manometer tube, compression pad and silicone-free syringes) (Figure 1b ) , and the following items that are not supplied: scissors, pen, sterile alcoholic wipes, timer and gloves (Figure 1c ) .
Open the VARITHENA Bi-Canister box and remove the VARITHENA Bi-Canister pouch. Open the Administration Pack and remove the components. Inspect the pouch and components for damage (do not use product if there are any visible signs of damage to pouch or components).
Unpacking VARITHENA:
Option B for 180 mg/18 mL Product: Convenience Box (Bi-Canister Box + 3 Ancillary Packs + 3 VARITHENA transfer units)
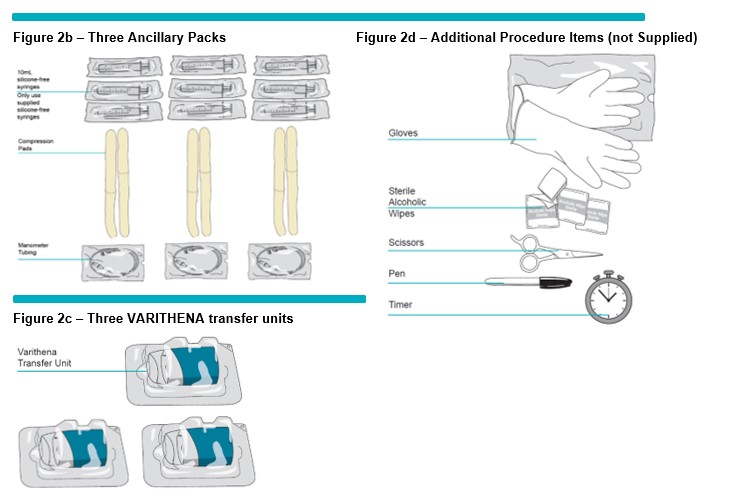
Gather all the items needed for the generation of foam: the VARITHENA Bi-Canister Box ) (Figure 2a ) , Ancillary Pack (including silicone-free syringes, manometer tubing, and compression pads (Figure 2b ) and VARITHENA transfer unit (Figure 2c ) , and the following items that are not supplied: scissors, pen, sterile alcoholic wipes, timer and gloves (Figure 2d ) .
Open the VARITHENA Convenience box and remove all the components. Open the VARITHENA Bi-Canister box and remove the VARITHENA Bi-Canister pouch. Open an Ancillary Pack and remove the components. Inspect the pouch and components for damage (do not use product if there are any visible signs of damage to pouch or components).
Unpacking VARITHENA:
Option B for 77.5 mg/7.75 mL product: Convenience Box (Bi-Canister Box + 1 Ancillary Pack + 1 VARITHENA transfer unit)
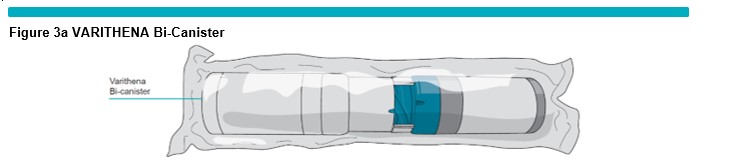
Gather all the items needed for the generation of foam: the VARITHENA Bi-Canister Box (Figure 3a ) , Ancillary Pack (including silicone-free syringes, manometer tubing, and compression pads (Figure 3b ) and VARITHENA transfer unit (Figure 3c ) , and the following items that are not supplied: scissors, pen, sterile alcoholic wipes, timer and gloves (Figure 3d ) .
Open the VARITHENA Convenience box and remove all the components. Open the VARITHENA Bi-Canister box and remove the VARITHENA Bi-Canister pouch. Open the Ancillary Pack and remove the components. Inspect the pouch and components for damage (do not use product if there are any visible signs of damage to pouch or components).
Preparing the Patient
Preparations for treating the patient with VARITHENA should include the following steps:
- Position the patient comfortably on the treatment table in a supine position with their hip externally rotated to facilitate access to the GSV.
- Use ultrasound to find the best site for venous access.
- Using an aseptic technique, infiltrate the skin over the venous access point with local anesthetic.
- Obtain venous access under ultrasound guidance.
- IV catheters that are 16 to 22 gauge and 40 - to 50 - mm long or micropuncture sets are recommended for venous access.
- Prefill the manometer tube with sterile heparinized normal saline solution and connect to the IV catheter.
- Confirm venous access by aspirating with a syringe, blood should be dark and under low pressure.
- Flush the IV catheter and manometer tube with heparinized normal saline and secure it to the skin with adhesive tape, leave the saline syringe connected.
- With the IV catheter in place and secure, place the patient supine and elevate the leg to approximately 45 degrees.
Complete all preparation of the patient and preparations for VARITHENA injectable foam injection before generation of the foam.
VARITHENA Preparation
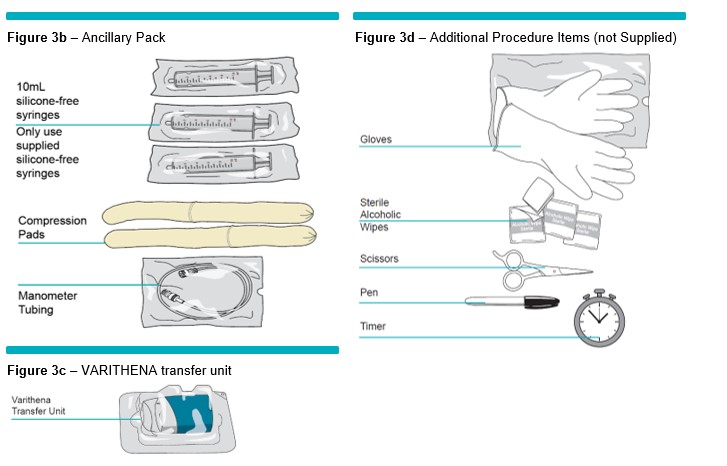
1 Wearing appropriate sterile gloves, open Bi-Canister pouch using a pair of scissors. Place canisters upright on a cleaned (sterile wipes) stable surface with the white oxygen canister on top (Figure 4 ) . Discard empty pouch.
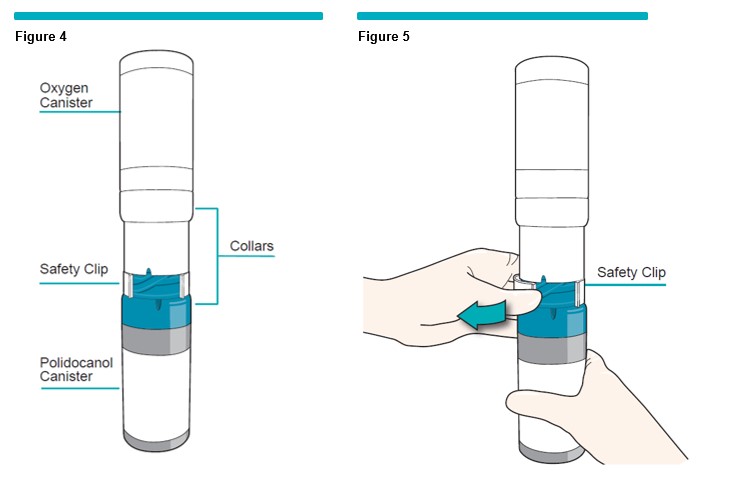
2 Remove the safety clip by lifting one corner of the clip out (Figure 5 ) . Discard the safety clip.
Gas Activation of the VARITHENA Canistern
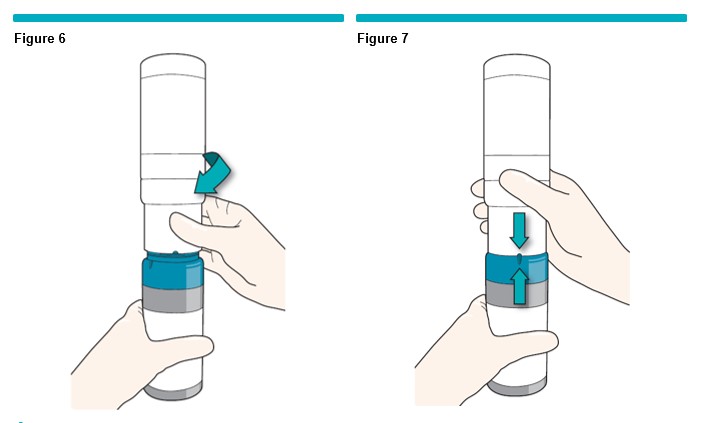
3 To begin the gas transfer, twist the canisters together clockwise (Figure 6 ) until they come to a stop and the small indicators/marks on the collars are aligned (Figure 7 ) . You may hear a bubbling sound.
While the canisters are activating, keep them upright on the clean flat surface for 1 minute. Use a timing device to keep track of the 1 minute time. Extended gassing periods (more than 10 minutes) are undesirable.
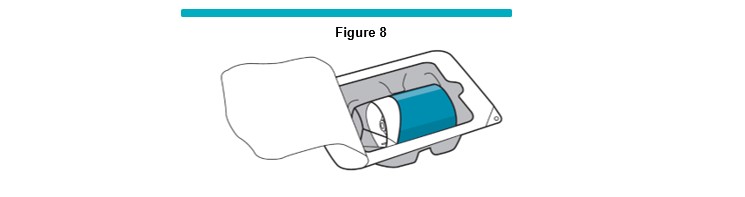
4 Note: In order to maintain sterility of the VARITHENA transfer unit, the following steps must be followed. While waiting 1 minute for the gas transfer, open a new VARITHENA transfer unit, blister pack, but leave the VARITHENA transfer unit in the package (Figure 8 ) .
The manometer tubing (20 inch) should have been previously filled with sterile heparinized normal saline solution.
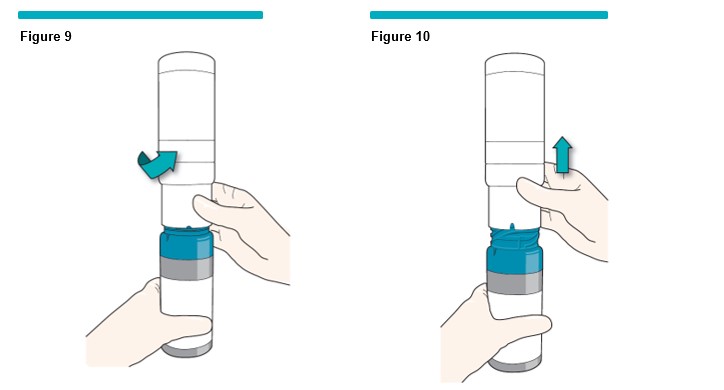
5 After 1 minute,
- Twist the two canisters by turning them in the opposite direction (counterclockwise) as before (Figure 9 ) .
- Pull straight up to separate the oxygen canister from the VARITHENA canister, as shown (Figure 10 ) . Do not separate canisters until you have a VARITHENA transfer unit ready to place onto the VARITHENA canister (See step 6 ).
- Put the oxygen canister (with white collar) aside.
- The VARITHENA canister (with blue collar) should remain on a clean flat surface, in the upright position.
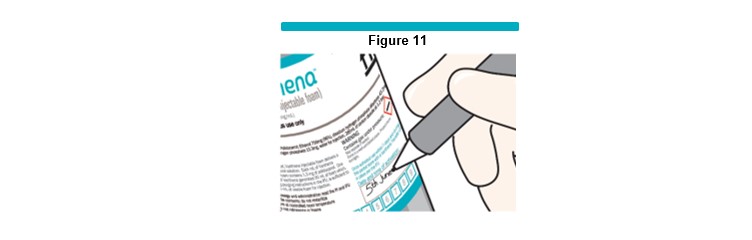
Write today’s date and time in the “Date and Time of Activation” box on the VARITHENA canister (Figure 11 )
Connecting a new VARITHENA transfer unit and syringe
6 Remove the VARITHENA transfer unit from the blister pack, wearing a fresh pair of sterile gloves. Make sure not to touch the sterile underside of the VARITHENA transfer unit, (discard VARITHENA transfer unit if contaminated).
Immediately place the VARITHENA transfer unit on top of the blue VARITHENA canister. Gently rotate the VARITHENA transfer unit clockwise as indicated (Figure 12 ) until it drops into the collar threads then twist the VARITHENA transfer unit (clockwise) until it reaches a stop (Figure 13 ).
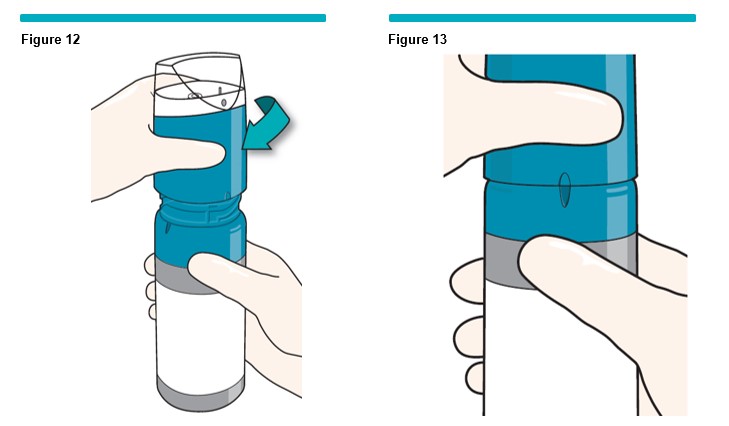
The system is now activated and ready for use.
7 Once all preparations for injection are complete, i.e., cannula in situ, patient’s leg elevated and a good ultrasound view of the saphenofemoral junction (SFJ) obtained, foam may be generated for immediate use.
Open a sterile 10 mL silicone-free syringe blister pack and keep it in the package until needed.
Remove the syringe from the package, and connect it to the VARITHENA transfer unit as shown (Figure 14 ).
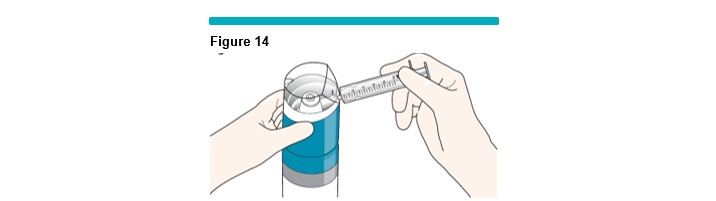
Priming a New Syringe
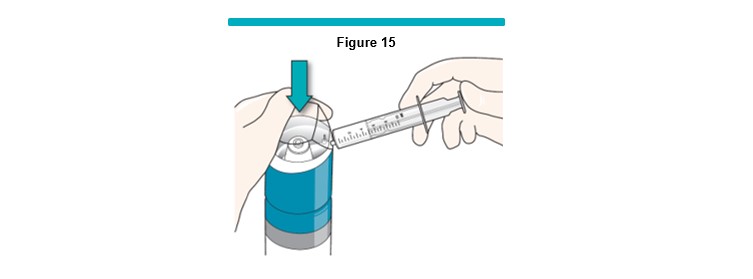
8 Gently press down the VARITHENA transfer unit to begin producing foam (Figure 15 ).
Using continuous pressure, allow the silicone-free syringe to fill between 3 mL and 5 mL.
Release the pressure on the VARITHENA transfer unit and leave the syringe connected.
9 Push the silicone-free syringe plunger in fully to discard its contents (Figure 16 ). Do not disconnect the syringe.
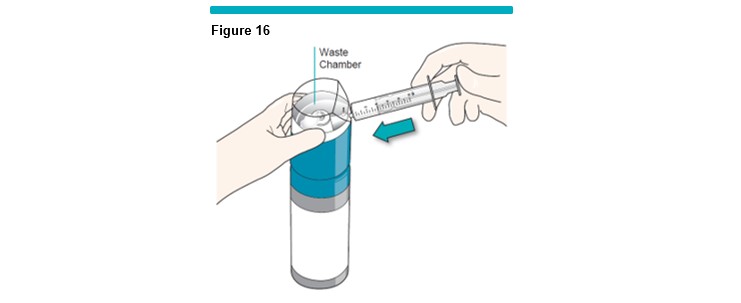
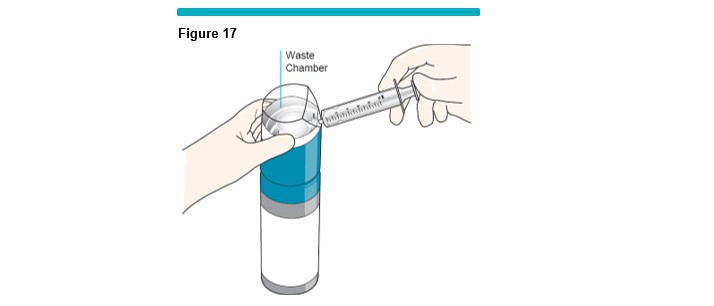
Note: The foam will automatically be diverted into the waste chamber within the VARITHENA transfer unit (Figure 17 ) . This process eliminates the small quantity of air in the syringe and VARITHENA transfer unit.
Generation of Foam
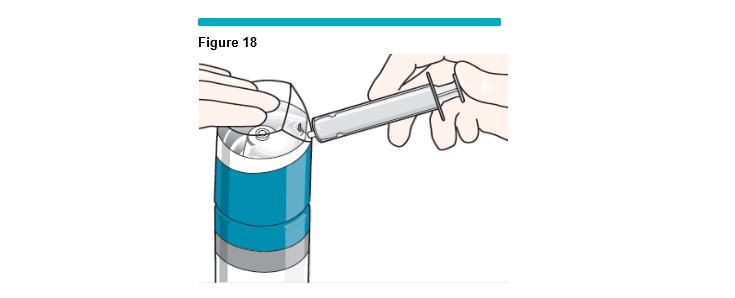
10 Foam Generation: The technique to produce usable foam requires a single purge cycle before filling the syringe, a process that takes less than 1 second.
While holding the silicone-free syringe plunger in place, gently press down on the VARITHENA transfer unit to begin the purge cycle (Figure 18 ).
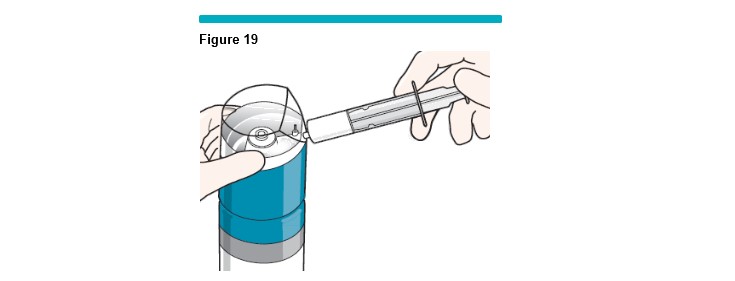
Visually inspect the flowing foam inside the VARITHENA transfer unit to make sure the visible air bubbles have been expelled (less than 1 second) before releasing the syringe plunger and allowing it to fill to the desired volume (Figure 19 ).
Draw up to 5 mL of foam into the syringe.
Inspecting and Injecting Foam
11 After the silicone-free syringe has filled to the desired volume, wait 10 seconds to allow the pressure to equalize before removing the syringe from the VARITHENA transfer unit (Figure 20 ).
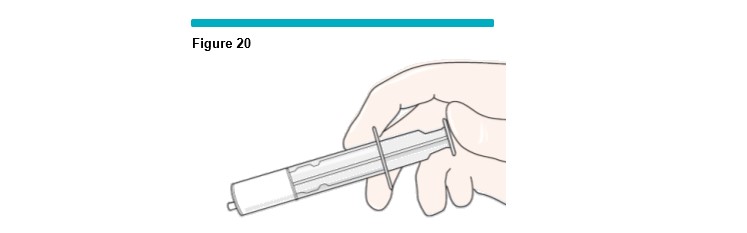
WARNING: As the foam fills the syringe and before injecting, inspect the syringe full of foam for any visible bubbles (easily seen with the unaided eye at arm’s length). If there are any present, empty the foam into the VARITHENA transfer unit waste chamber and refill the syringe.
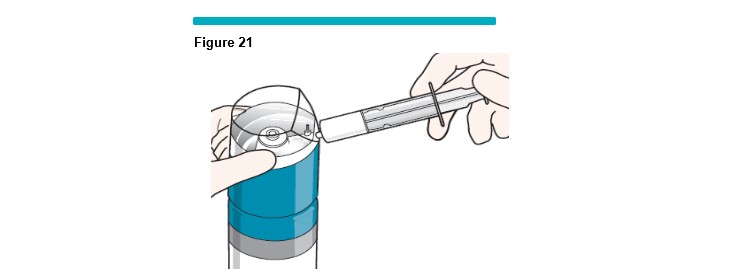
12 Remove the syringe from the VARITHENA transfer unit and inspect it for visible bubbles (Figure 21 ) .
If no visible bubbles are present then the foam is ready for use.
Use the foam within 75 seconds of generation or discard and generate new foam.
WARNING: The total amount of foam injected in any one treatment session must not exceed 15 mL, comprised of individual injections of up to 5 mL each.
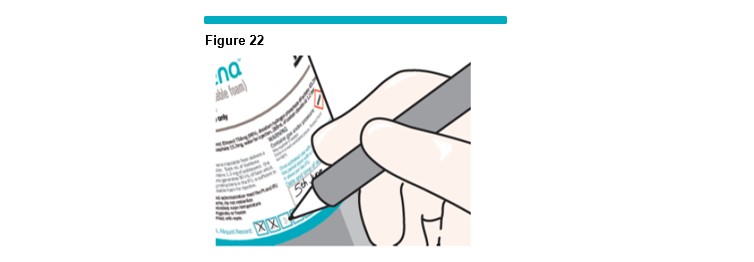
After each treatment session, mark-off on the canister label the number of aliquots of up to 5 mL of usable foam drawn from the canister per step 11 (Figure 22 ).
13 Connect a syringe of freshly generated foam to the manometer tubing, which is already connected to the cannula, in preparation for the initial injection. The manometer tubing (20) inch should have been previously filled with sterile heparinized normal saline solution.
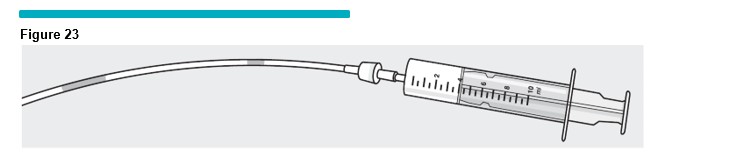
14 Inject the foam at approximately 0.5 mL to 1.0 mL per second through the manometer tubing. Five (5) mLs of foam should be injected in approximately 10 seconds. Always inspect the foam as it passes through the manometer tubing for visible bubbles (Figure 23 ). If any visible bubbles are seen (easily seen with the unaided eye at arm's length) they should be aspirated back into the silicone-free syringe and the syringe contents discarded back into the VARITHENA transfer unit waste chamber, and a fresh syringe of foam generated.
Notes: Use a new sterile syringe after each injection.
WARNING: The total amount of foam injected in any one treatment session must not exceed 15 mL, comprised of individual injections of up to 5 mL each
Do not remove VARITHENA transfer unit if the VARITHENA canister is to be stored (see Storage)
Change sterile gloves appropriately, to limit any contamination of the VARITHENA transfer unit and Bi-Canister.
Compression Pads
15 Once treatment is complete, the Compression Pads should be used:
The objective of the pads is to focus the compression forces on the treated vein to keep them as free from blood as possible, thus minimizing retained thrombus.
The compression pads supplied should be placed along the course of the treated trunk vein in the thigh, and over raised treated varicose veins above and below the knee. The pads may be shaped to follow the course of the veins. The pads should be placed outside the first layer of limited stretch bandage and held in place by a second layer of bandage.
The appropriate length compression stocking is then applied.
Replacing the VARITHENA Transfer Unit
Important Note: Do not replace the VARITHENA transfer unit if the canister is to be stored for future use. The activated VARITHENA canister should always be stored in an appropriately cleaned area with a VARITHENA transfer unit in place in the upright position at or below 86°F (30°C), do not refrigerate or freeze. Replace the VARITHENA transfer unit just prior to the next treatment session.
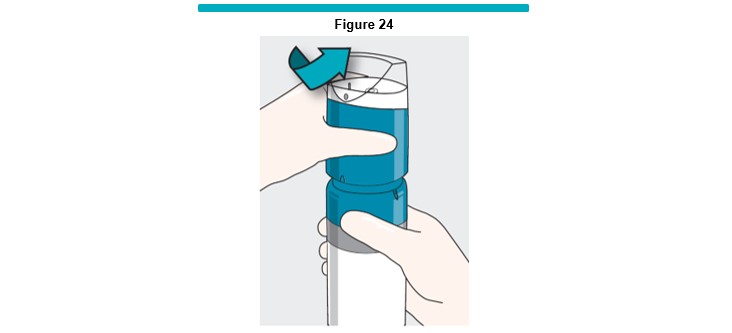
16 Wearing appropriate new sterile gloves, hold the VARITHENA canister, twist the VARITHENA transfer unit counterclockwise and then pull up to separate from the canister (Figure 24 ).
17 Discard the old VARITHENA transfer unit and open a new VARITHENA transfer unit.
Make sure not to touch the sterile underside of the VARITHENA transfer unit.
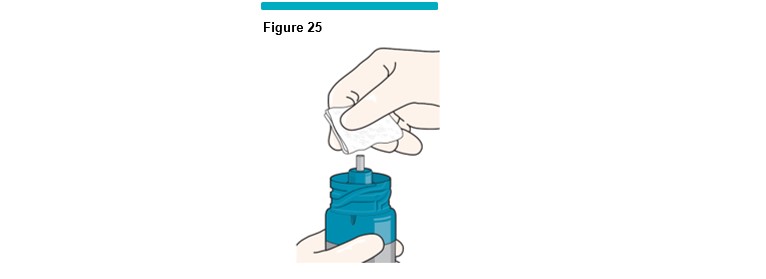
18 Swab the uncovered shuttle with a fresh sterile alcohol wipe (Figure 25 ) and immediately place the VARITHENA transfer unit on top of the VARITHENA canister.
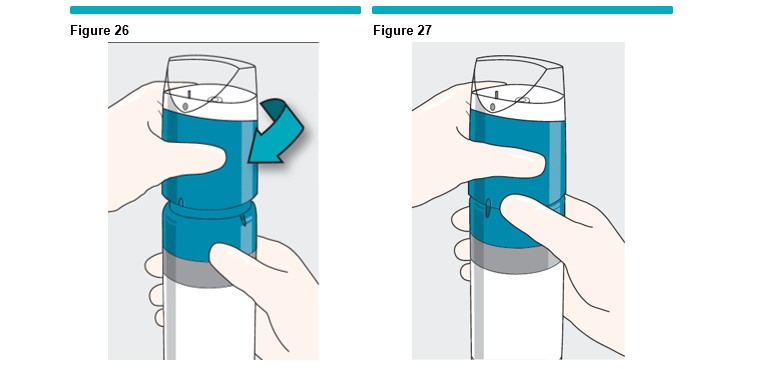
Gently rotate the VARITHENA transfer unit clockwise until it drops into the collar threads (Figure 26 ) , then twist the VARITHENA transfer unit (clockwise) until it reaches a stop (Figure 27 ) .
The VARITHENA device now ready for use for a new treatment session, following the instructions in Steps 7 to 15.
Storage and Disposal
Note: The activated VARITHENA canister should always be stored with a VARITHENA transfer unit in place in the upright position at or below 86°F (30°C) - do not refrigerate or freeze, in an appropriately controlled clean area to limit contamination.
Dispose of VARITHENA and oxygen canisters following local and state regulations for aerosol disposal.
The VARITHENA transfer unit can be disposed of as non-toxic non-clinical waste.
| 180 mg/18 mL Configuration: | 77.5 mg/7.75 mL Configuration: |
| Once the VARITHENA canister has been activated, the shelf life for the product is thirty (30) calendar days. Always write the activation date and time on the canister and verify the product has not expired prior to use. Net Contents:18 mL One canister of VARITHENA in the 180 mg/18 mL Configuration contains: 180 mg Polidocanol, 756 mg alcohol (96% v/v), 43.2 mg disodium hydrogen phosphate dihydrate, 15.3 mg potassium dihydrogen phosphate, water for injection. One canister of VARITHENA generates 90 mL of foam which, following purging instructions contained in this IFU, is sufficient to yield 45 mL of usable foam for injection. The gas mix of the foam is 65:35 O 2 :CO 2 . NDC 60635-118-01 VARITHENA 180 mg/18 mL Bi- Canister Administration Pack NDC 60635-133-01 VARITHENA 180 mg/18 mL Convenience Pack | Once the VARITHENA canister has been activated, the canister must be used within thirty (30) calendar days. Always write the activation date and time on the canister and verify the product has not expired prior to use. Net Contents:7.75 mL One canister of VARITHENA in the 77.5 mg/7.75 mL Configuration contains: 77.5 mg Polidocanol, 325.5 mg alcohol (96% v/v), 18.6 mg disodium hydrogen phosphate dihydrate, 6.6 mg potassium dihydrogen phosphate, water for injection. One canister of VARITHENA generates 30 mL of foam which, following purging instructions contained in this IFU, is sufficient to yield 15 mL of usable foam for injection. The gas mix of the foam is 65:35 O 2 :CO 2 . NDC 60635-107-01 VARITHENA 77.5 mg/7.75 mL Bi- Canister Administration Pack NDC 60635-111-01 VARITHENA 77.5 mg/7.75 mL Convenience Pack |
Manufactured for Provensis Ltd by: Biocompatibles UK Ltd Chapman House, Weydon Lane, Farnham, Surrey, UK, GU9 8QL Distributed by: Biocompatibles Inc.
VARITHENA is a registered trademark of Provensis Ltd BTG and the BTG roundel logo are registered trademarks of BTG International Ltd Provensis Ltd, Biocompatibles UK Ltd, and Biocompatibles Inc. are BTG International group companies
Mechanism of Action
VARITHENA is a drug/device combination product that generates injectable foam. The injectable foam is composed of a liquid and gas phase, both of which are necessary to have its therapeutic effect. VARITHENA is intended to act as follows: (1) the foam displaces blood from the vein to be treated, and (2) the polidocanol then scleroses the endothelium.
The active pharmaceutical ingredient of VARITHENA is polidocanol, a non-ionic surfactant sclerosing agent. The hydrophobic pole of the polidocanol molecule attaches to the lipid cell membrane of the venous endothelium, resulting in disruption of the osmotic barrier, destruction of the venous endothelium, and vasospasm. Following exposure to polidocanol, the interior surface of the vein becomes thrombogenic, which leads to thrombus formation and venous occlusion. The occluded vein is eventually replaced by fibrous connective tissue. Polidocanol is deactivated upon contact with blood, thus limiting the sclerosant action to the endothelium near the site of injection.