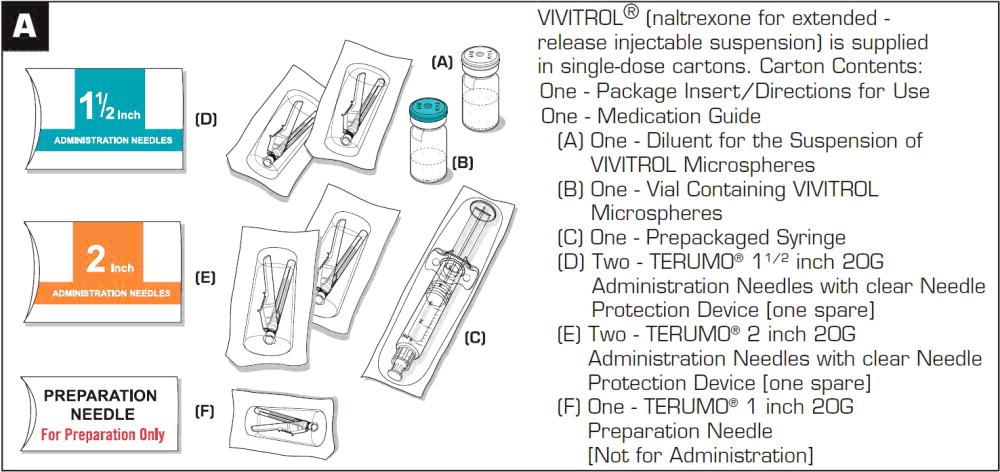
Get your patient on Vivitrol (Naltrexone)
Vivitrol prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Vivitrol patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- VIVITROL must be prepared and administered by a healthcare provider (2.1 , 2.6 ).
- Prior to initiating VIVITROL, an opioid-free duration of a minimum of 7–10 days is recommended for patients, to avoid precipitation of opioid withdrawal that may be severe enough to require hospitalization (2.1 ).
- The recommended dose of VIVITROL is 380 mg delivered intramuscularly (deep) as a gluteal injection, every 4 weeks or once a month, alternating buttocks for each subsequent injection, using the carton components provided (2.1 ).
- VIVITROL must ONLY be administered as a deep intramuscular gluteal injection (2.1 ).
- See Full Prescribing Information for complete Directions for Use (2.6 ).
Important Dosage and Administration Information
VIVITROL must be prepared and administered by a healthcare provider.
VIVITROL must ONLY be administered as a deep intramuscular gluteal injection.
Parenteral products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit. A properly mixed suspension will be milky white, will not contain clumps, and will move freely down the wall of the vial [see Dosage and Administration (2.6 )] .
Prior to initiating VIVITROL, an opioid-free duration of a minimum of 7–10 days is recommended for patients, to avoid precipitation of opioid withdrawal that may be severe enough to require hospitalization [see Warnings and Precautions (5.3 )].
The recommended dose of VIVITROL is 380 mg delivered intramuscularly (deep) as a gluteal injection every 4 weeks or once a month, alternating buttocks for each subsequent injection, using the carton components provided [see How Supplied/Storage and Handling (16 )] . The needles provided in the carton are customized needles. VIVITROL must not be injected using any other needle. The needle lengths (either 1 1/2 or 2 inches) may not be adequate in every patient because of body habitus. Body habitus should be assessed prior to each injection for each patient to assure that needle length is adequate for intramuscular administration. For patients with a larger amount of subcutaneous tissue overlying the gluteal muscle, the administering healthcare provider may utilize the supplied 2-inch needle with needle protection device to help ensure that the injectate reaches the intramuscular mass. For very lean patients, the 1 1/2-inch needle may be appropriate to prevent the needle contacting the periosteum. Either needle may be used for patients with average body habitus. Healthcare providers should ensure that the VIVITROL injection is given correctly, and should consider alternate treatment for those patients whose body habitus precludes an intramuscular gluteal injection with one of the provided needles.
If a patient misses a dose, he/she should be instructed to receive the next dose as soon as possible.
Pretreatment with oral naltrexone is not required before using VIVITROL.
Patient Access to an Opioid Reversal Agent for the Emergency Treatment of Opioid Overdose
At the initial VIVITROL injection and with each subsequent injection, inform patients and caregivers about opioid overdose reversal agents (e.g., naloxone, nalmefene) and discuss the importance of having access to an opioid overdose reversal agent. Because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose after VIVITROL treatment is discontinued, at the end of the VIVITROL dosing interval (i.e., near the end of the month that VIVITROL was administered), or after a dose of VIVITROL is missed, strongly consider recommending or prescribing an overdose reversal agent for the emergency treatment of opioid overdose [see Warnings and Precautions (5.1 )] .
Discuss the options for obtaining an opioid overdose reversal agent (e.g., prescription, over-the-counter, or as part of a community-based program [see Warning and Precautions (5.1 )] .
There are important differences among the opioid overdose reversal agents, such as route of administration, product strength, approved patient age range, and pharmacokinetics. Be familiar with these differences, as outlined in the approved labeling for those products, prior to recommending or prescribing such an agent.
Reinitiation of Treatment in Patients Previously Discontinued
Switching From Oral Naltrexone
There are no systematically collected data that specifically address the switch from oral naltrexone to VIVITROL.
Switching from Buprenorphine, Buprenorphine/Naloxone, or Methadone
There are no systematically collected data that specifically address the switch from buprenorphine or methadone to VIVITROL; however, review of postmarketing case reports have indicated that some patients may experience severe manifestations of precipitated withdrawal when being switched from opioid agonist therapy to opioid antagonist therapy [see Warnings and Precautions (5.3 )] . Patients transitioning from buprenorphine or methadone may be vulnerable to precipitation of withdrawal symptoms for as long as 2 weeks. Healthcare providers should be prepared to manage withdrawal symptomatically with non-opioid medications.
Directions for Use
VIVITROL must be prepared and administered by a healthcare provider.
VIVITROL must ONLY be administered as a deep intramuscular gluteal injection.
To ensure proper dosing, it is important that you follow the preparation and administration instructions outlined in this document.
VIVITROL must be suspended only in the diluent supplied in the carton and must be administered only with one of the administration needles supplied in the carton. The microspheres, diluent, preparation needle, and an administration needle with needle protection device are required for preparation and administration. Two thin-walled 1 1/2-inch needles with needle protection device and two 2-inch thin-walled needles with needle protection device have been provided to accommodate varying patient body habitus. For patients with a larger amount of subcutaneous tissue overlying the gluteal muscle, the administering healthcare provider may utilize the supplied 2-inch needle with needle protection device to help ensure that the injectate reaches the intramuscular mass. For very lean patients, the 1 1/2-inch needle may be appropriate to prevent the needle contacting the periosteum. Either needle may be used for patients with average body habitus. A spare administration needle of each size is provided in case of clogging [see How Supplied/Storage and Handling (16 )] . Do not substitute any other components for the components of the carton.
Prior to preparation, allow drug to reach room temperature (approximately 45 minutes).
Parenteral products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit. A properly mixed suspension will be milky white, will not contain clumps, and will move freely down the wall of the vial [see Directions for Use, illustration below ] .
Keep out of reach of children.
Prepare and administer the VIVITROL suspension using aseptic technique.
WARNING: To reduce the risk of a needlestick:
- Do not intentionally disengage the needle protection device.
- Discard bent or damaged needle into a sharps container and use the spare needle provided. Do not attempt to straighten the needle or engage needle protection device if the needle is bent or damaged.
- Do not mishandle the needle protection device in a way that could lead to protrusion of the needle.
- Do not use free hand to press sheath over needle.
THE CARTON SHOULD NOT BE EXPOSED TO TEMPERATURES EXCEEDING 25 °C (77 °F).
The entire carton should be stored in the refrigerator (2 °C to 8 °C, 36 °F to 46 °F). Unrefrigerated, VIVITROL microspheres can be stored at temperatures not exceeding 25 °C (77 °F) for no more than 7 days prior to administration. Do not expose unrefrigerated product to temperatures above 25 °C (77 °F). VIVITROL should not be frozen.
Parenteral products should be visually inspected for particulate matter and discoloration prior to administration. | |
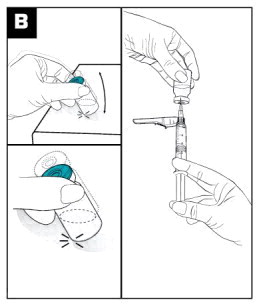 |
|
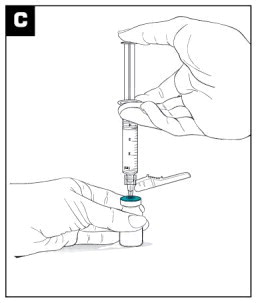 | Inject the 3.4 mL of diluent into the VIVITROL microsphere vial. (see Figure C ) |
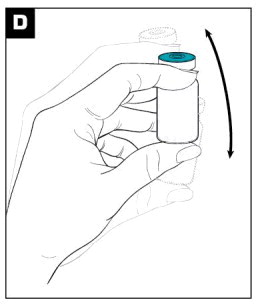 | Mix the powder and diluent by vigorously shaking the vial for approximately 1 minute. (see Figure D ) Ensure that the dose is thoroughly suspended prior to proceeding to Step E. A PROPERLY MIXED SUSPENSION WILL BE MILKY WHITE, WILL NOT CONTAIN CLUMPS, AND WILL MOVE FREELY DOWN THE WALLS OF THE VIAL. |
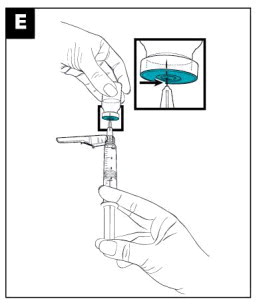 |
|
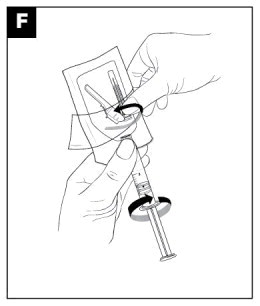 |
|
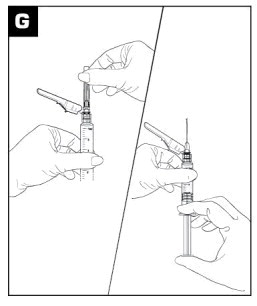 |
|
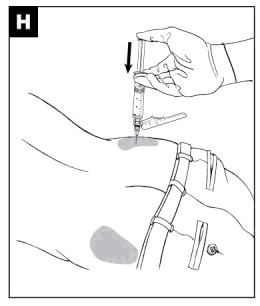 |
|
 | After the injection is administered, cover the needle by pressing the needle protection device against a flat surface using a one-handed technique to activate the safety mechanism away from self and others. (see Figure I ) |
 | Visually confirm needle is fully engaged into the needle protection device. (see Figure J ) DISPOSE OF USED AND UNUSED ITEMS IN PROPER WASTE CONTAINERS. |

By using PrescriberAI, you agree to the AI Terms of Use.
Vivitrol prescribing information
INDICATIONS AND USAGE
Treatment with VIVITROL should be part of a comprehensive management program that includes psychosocial support.
Alcohol Dependence
VIVITROL is indicated for the treatment of alcohol dependence in patients who are able to abstain from alcohol in an outpatient setting prior to initiation of treatment with VIVITROL. Patients should not be actively drinking at the time of initial VIVITROL administration.
Opioid Dependence
VIVITROL is indicated for the prevention of relapse to opioid dependence, following opioid detoxification.
DOSAGE AND ADMINISTRATION
- VIVITROL must be prepared and administered by a healthcare provider (2.1 , 2.6 ).
- Prior to initiating VIVITROL, an opioid-free duration of a minimum of 7–10 days is recommended for patients, to avoid precipitation of opioid withdrawal that may be severe enough to require hospitalization (2.1 ).
- The recommended dose of VIVITROL is 380 mg delivered intramuscularly (deep) as a gluteal injection, every 4 weeks or once a month, alternating buttocks for each subsequent injection, using the carton components provided (2.1 ).
- VIVITROL must ONLY be administered as a deep intramuscular gluteal injection (2.1 ).
- See Full Prescribing Information for complete Directions for Use (2.6 ).
Important Dosage and Administration Information
VIVITROL must be prepared and administered by a healthcare provider.
VIVITROL must ONLY be administered as a deep intramuscular gluteal injection.
Parenteral products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit. A properly mixed suspension will be milky white, will not contain clumps, and will move freely down the wall of the vial [see Dosage and Administration (2.6 )] .
Prior to initiating VIVITROL, an opioid-free duration of a minimum of 7–10 days is recommended for patients, to avoid precipitation of opioid withdrawal that may be severe enough to require hospitalization [see Warnings and Precautions (5.3 )].
The recommended dose of VIVITROL is 380 mg delivered intramuscularly (deep) as a gluteal injection every 4 weeks or once a month, alternating buttocks for each subsequent injection, using the carton components provided [see How Supplied/Storage and Handling (16 )] . The needles provided in the carton are customized needles. VIVITROL must not be injected using any other needle. The needle lengths (either 1 1/2 or 2 inches) may not be adequate in every patient because of body habitus. Body habitus should be assessed prior to each injection for each patient to assure that needle length is adequate for intramuscular administration. For patients with a larger amount of subcutaneous tissue overlying the gluteal muscle, the administering healthcare provider may utilize the supplied 2-inch needle with needle protection device to help ensure that the injectate reaches the intramuscular mass. For very lean patients, the 1 1/2-inch needle may be appropriate to prevent the needle contacting the periosteum. Either needle may be used for patients with average body habitus. Healthcare providers should ensure that the VIVITROL injection is given correctly, and should consider alternate treatment for those patients whose body habitus precludes an intramuscular gluteal injection with one of the provided needles.
If a patient misses a dose, he/she should be instructed to receive the next dose as soon as possible.
Pretreatment with oral naltrexone is not required before using VIVITROL.
Patient Access to an Opioid Reversal Agent for the Emergency Treatment of Opioid Overdose
At the initial VIVITROL injection and with each subsequent injection, inform patients and caregivers about opioid overdose reversal agents (e.g., naloxone, nalmefene) and discuss the importance of having access to an opioid overdose reversal agent. Because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose after VIVITROL treatment is discontinued, at the end of the VIVITROL dosing interval (i.e., near the end of the month that VIVITROL was administered), or after a dose of VIVITROL is missed, strongly consider recommending or prescribing an overdose reversal agent for the emergency treatment of opioid overdose [see Warnings and Precautions (5.1 )] .
Discuss the options for obtaining an opioid overdose reversal agent (e.g., prescription, over-the-counter, or as part of a community-based program [see Warning and Precautions (5.1 )] .
There are important differences among the opioid overdose reversal agents, such as route of administration, product strength, approved patient age range, and pharmacokinetics. Be familiar with these differences, as outlined in the approved labeling for those products, prior to recommending or prescribing such an agent.
Reinitiation of Treatment in Patients Previously Discontinued
Switching From Oral Naltrexone
There are no systematically collected data that specifically address the switch from oral naltrexone to VIVITROL.
Switching from Buprenorphine, Buprenorphine/Naloxone, or Methadone
There are no systematically collected data that specifically address the switch from buprenorphine or methadone to VIVITROL; however, review of postmarketing case reports have indicated that some patients may experience severe manifestations of precipitated withdrawal when being switched from opioid agonist therapy to opioid antagonist therapy [see Warnings and Precautions (5.3 )] . Patients transitioning from buprenorphine or methadone may be vulnerable to precipitation of withdrawal symptoms for as long as 2 weeks. Healthcare providers should be prepared to manage withdrawal symptomatically with non-opioid medications.
Directions for Use
VIVITROL must be prepared and administered by a healthcare provider.
VIVITROL must ONLY be administered as a deep intramuscular gluteal injection.
To ensure proper dosing, it is important that you follow the preparation and administration instructions outlined in this document.
VIVITROL must be suspended only in the diluent supplied in the carton and must be administered only with one of the administration needles supplied in the carton. The microspheres, diluent, preparation needle, and an administration needle with needle protection device are required for preparation and administration. Two thin-walled 1 1/2-inch needles with needle protection device and two 2-inch thin-walled needles with needle protection device have been provided to accommodate varying patient body habitus. For patients with a larger amount of subcutaneous tissue overlying the gluteal muscle, the administering healthcare provider may utilize the supplied 2-inch needle with needle protection device to help ensure that the injectate reaches the intramuscular mass. For very lean patients, the 1 1/2-inch needle may be appropriate to prevent the needle contacting the periosteum. Either needle may be used for patients with average body habitus. A spare administration needle of each size is provided in case of clogging [see How Supplied/Storage and Handling (16 )] . Do not substitute any other components for the components of the carton.
Prior to preparation, allow drug to reach room temperature (approximately 45 minutes).
Parenteral products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit. A properly mixed suspension will be milky white, will not contain clumps, and will move freely down the wall of the vial [see Directions for Use, illustration below ] .
Keep out of reach of children.
Prepare and administer the VIVITROL suspension using aseptic technique.
WARNING: To reduce the risk of a needlestick:
- Do not intentionally disengage the needle protection device.
- Discard bent or damaged needle into a sharps container and use the spare needle provided. Do not attempt to straighten the needle or engage needle protection device if the needle is bent or damaged.
- Do not mishandle the needle protection device in a way that could lead to protrusion of the needle.
- Do not use free hand to press sheath over needle.
THE CARTON SHOULD NOT BE EXPOSED TO TEMPERATURES EXCEEDING 25 °C (77 °F).
The entire carton should be stored in the refrigerator (2 °C to 8 °C, 36 °F to 46 °F). Unrefrigerated, VIVITROL microspheres can be stored at temperatures not exceeding 25 °C (77 °F) for no more than 7 days prior to administration. Do not expose unrefrigerated product to temperatures above 25 °C (77 °F). VIVITROL should not be frozen.

| Parenteral products should be visually inspected for particulate matter and discoloration prior to administration. | |
 |
|
 | Inject the 3.4 mL of diluent into the VIVITROL microsphere vial. (see Figure C ) |
 | Mix the powder and diluent by vigorously shaking the vial for approximately 1 minute. (see Figure D ) Ensure that the dose is thoroughly suspended prior to proceeding to Step E. A PROPERLY MIXED SUSPENSION WILL BE MILKY WHITE, WILL NOT CONTAIN CLUMPS, AND WILL MOVE FREELY DOWN THE WALLS OF THE VIAL. |
 |
|
 |
|
 |
|
 |
|
 | After the injection is administered, cover the needle by pressing the needle protection device against a flat surface using a one-handed technique to activate the safety mechanism away from self and others. (see Figure I ) |
 | Visually confirm needle is fully engaged into the needle protection device. (see Figure J ) DISPOSE OF USED AND UNUSED ITEMS IN PROPER WASTE CONTAINERS. |
DOSAGE FORMS AND STRENGTHS
VIVITROL is an off-white to light tan powder for injectable suspension in a 5 mL single-dose vial.
VIVITROL contains 380 mg of naltrexone in a microsphere formulation per vial (337 mg of naltrexone per gram of microspheres).
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
The available data from published case series with VIVITROL use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There are clinical considerations (see Clinical Considerations ). Reproduction and developmental animal studies have not been conducted for VIVITROL. Daily oral administration of naltrexone to female rats and rabbits increased the incidence of early fetal loss at exposures ≥ 11 times and ≥ 2 times the human exposure, respectively. Daily oral administration of naltrexone to pregnant rats and rabbits during the period of organogenesis did not induce malformation at exposures up to 175 times and 14 times the human exposure, respectively (see Data ).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo-fetal risk
Untreated opioid addiction in pregnancy is associated with adverse obstetrical outcomes such as low birth weight, preterm birth, and fetal death. In addition, untreated opioid addiction often results in continued or relapsing illicit opioid use.
Published studies have demonstrated that alcohol is associated with fetal harm including growth restriction, facial abnormalities, central nervous system abnormalities, behavioral disorders, and impaired intellectual development.
Data
Animal Data
Reproduction and developmental studies have not been conducted for VIVITROL. Studies with naltrexone administered via the oral route have been conducted in pregnant rats and rabbits.
Daily oral administration of naltrexone has been shown to increase the incidence of early fetal loss when given to rats at doses ≥30 mg/kg/day (11 times the human exposure based on an AUC (0-28d) comparison) and to rabbits at oral doses ≥60 mg/kg/day (2 times the human exposure based on an AUC (0-28d) comparison).
Daily oral administration of naltrexone to rats and rabbits during the period of organogenesis did not induce malformations at doses up to 200 mg/kg/day (175- and 14-times the human exposure based on an AUC (0-28d) comparison, respectively).
Lactation
Risk Summary
Naltrexone and its major metabolite, 6β-naltrexol, are present in human milk. There are no data on the effects on the breastfed infant or the effects on milk production. The developmental health benefits of breastfeeding should be considered along with the mother's clinical need for naltrexone and any potential adverse effects on the breastfed infant from naltrexone or the mother's underlying maternal condition.
Pediatric Use
The safety and efficacy of VIVITROL have not been established in the pediatric population. The pharmacokinetics of VIVITROL have not been evaluated in a pediatric population.
Geriatric Use
In trials of alcohol-dependent subjects, 2.6% (n=26) of subjects were >65 years of age, and one patient was >75 years of age. Clinical studies of VIVITROL did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger subjects. No subjects over age 65 were included in studies of opioid-dependent subjects. The pharmacokinetics of VIVITROL have not been evaluated in the geriatric population.
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.
Renal Impairment
Pharmacokinetics of VIVITROL are not altered in subjects with mild renal insufficiency (creatinine clearance of 50-80 mL/min). Dose adjustment is not required in patients with mild renal impairment. VIVITROL pharmacokinetics have not been evaluated in subjects with moderate and severe renal insufficiency. Because naltrexone and its primary metabolite are excreted primarily in the urine, caution is recommended in administering VIVITROL to patients with moderate to severe renal impairment [see Clinical Pharmacology (12.3 )] .
Hepatic Impairment
The pharmacokinetics of VIVITROL are not altered in subjects with mild to moderate hepatic impairment (Groups A and B of the Child-Pugh classification). Dose adjustment is not required in subjects with mild or moderate hepatic impairment. VIVITROL pharmacokinetics were not evaluated in subjects with severe hepatic impairment [see Clinical Pharmacology (12.3 )] .
CONTRAINDICATIONS
VIVITROL is contraindicated in:
- Patients receiving opioid analgesics [see Warnings and Precautions (5.3 )] .
- Patients with current physiologic opioid dependence [see Warnings and Precautions (5.3 )] .
- Patients in acute opioid withdrawal [see Warnings and Precautions (5.3 )] .
- Any individual who has failed the naloxone challenge test or has a positive urine screen for opioids [see Warnings and Precautions (5.3 )] .
- Patients who have previously exhibited hypersensitivity to naltrexone, PLG, carboxymethylcellulose, or any other components of the diluent [see Warnings and Precautions (5.8 )].
WARNINGS AND PRECAUTIONS
- Vulnerability to Opioid Overdose : Following VIVITROL treatment, opioid tolerance is reduced from pretreatment baseline and patients are vulnerable to potentially fatal overdose at the end of a dosing interval, after missing a dose, or after discontinuing VIVITROL treatment. Attempts to overcome blockade may also lead to fatal overdose. Strongly consider recommending or prescribing an opioid reversal agent (e.g., naloxone, nalmefene) for the emergency treatment of opioid overdose (5.1 ).
- Injection Site Reactions : VIVITROL must be prepared and administered by a healthcare provider. In some cases, injection site reactions may be very severe. Some cases of injection site reactions required surgical intervention (5.2 ).
- Precipitation of Opioid Withdrawal : Opioid-dependent and opioid-using patients, including those being treated for alcohol dependence, should be opioid-free before starting VIVITROL treatment, and should notify healthcare providers of any recent opioid use. An opioid-free duration of a minimum of 7-10 days is recommended for patients to avoid precipitation of opioid withdrawal that may be severe enough to require hospitalization (5.3 ).
- Hepatotoxicity : Cases of hepatitis and clinically significant liver dysfunction were observed in association with VIVITROL treatment during the clinical development program and in the postmarketing period. Discontinue use of VIVITROL in the event of symptoms or signs of acute hepatitis (5.4 ).
- Depression and Suicidality : Monitor patients for the development of depression or suicidal thinking (5.5 ).
- When Reversal of VIVITROL Blockade Is Required for Pain Management : In an emergency situation in patients receiving VIVITROL, suggestions for pain management include regional analgesia or use of non-opioid analgesics (5.6 ).
- Eosinophilic Pneumonia: Patients who develop dyspnea and hypoxemia should seek medical attention immediately. Consider the possibility of eosinophilic pneumonia in patients who do not respond to antibiotics (5.7 ).
- Hypersensitivity Reactions Including Anaphylaxis: Cases of urticaria, angioedema, and anaphylaxis have been observed with the use of VIVITROL (5.8 ).
Vulnerability to Opioid Overdose
After opioid detoxification, patients are likely to have reduced tolerance to opioids. VIVITROL blocks the effects of exogenous opioids for approximately 28 days after administration. However, as the blockade wanes and eventually dissipates completely, patients who have been treated with VIVITROL may respond to lower doses of opioids than previously used, just as they would have shortly after completing detoxification. This could result in potentially life-threatening opioid intoxication (respiratory compromise or arrest, circulatory collapse, etc.) if the patient uses previously tolerated doses of opioids. Cases of opioid overdose with fatal outcomes have been reported in patients who used opioids at the end of a dosing interval, after missing a scheduled dose, or after discontinuing treatment.
Patients should be alerted that they may be more sensitive to opioids, even at lower doses, after VIVITROL treatment is discontinued, especially at the end of a dosing interval (i.e., near the end of the month that VIVITROL was administered), or after a dose of VIVITROL is missed. It is important that patients inform family members and the people closest to the patient of this increased sensitivity to opioids and the risk of overdose [see Patient Counseling Information (17 )].
There is also the possibility that a patient who is treated with VIVITROL could overcome the opioid blockade effect of VIVITROL. Although VIVITROL is a potent antagonist with a prolonged pharmacological effect, the blockade produced by VIVITROL is surmountable. The plasma concentration of exogenous opioids attained immediately following their acute administration may be sufficient to overcome the competitive receptor blockade. This poses a potential risk to individuals who attempt, on their own, to overcome the blockade by administering large amounts of exogenous opioids. Any attempt by a patient to overcome the antagonism by taking opioids is especially dangerous and may lead to life-threatening opioid intoxication or fatal overdose. Patients should be told of the serious consequences of trying to overcome the opioid blockade [see Patient Counseling Information (17 )] .
Patient Access to an Opioid Overdose Reversal Agent for the Emergency Treatment of Opioid Overdose
At the initial VIVITROL injection and with each subsequent injection, inform patients and caregivers about opioid overdose reversal agents (e.g., naloxone, nalmefene) and discuss the importance of having access to an opioid overdose reversal agent. Because of the risks for opioid overdose described above, both at the initial VIVITROL injection and with each subsequent injection, strongly consider recommending or prescribing an opioid overdose reversal agent for the emergency treatment of an opioid overdose.
Discuss the options for obtaining an opioid overdose reversal agent (e.g., prescription, over-the-counter, or as part of a community-based program).
There are important differences among the opioid overdose reversal agents, such as route of administration, product strength, approved patient age range, and pharmacokinetics. Be familiar with these differences, as outlined in the approved labeling for those products, prior to recommending or prescribing such an agent.
Educate patients and caregivers on how to recognize the signs and symptoms of an opioid overdose and how to use an opioid overdose reversal agent for the emergency treatment of opioid overdose. Emphasize the importance of calling 911 or getting emergency medical help in all cases of known or suspected opioid overdose, even if an opioid overdose reversal agent is administered.
Injection Site Reactions
VIVITROL must be prepared and administered by a healthcare provider.
VIVITROL must ONLY be administered as a deep intramuscular gluteal injection.
VIVITROL injections may be followed by pain, tenderness, induration, swelling, erythema, bruising, or pruritus; however, in some cases injection site reactions may be very severe. In the clinical trials, one patient developed an area of induration that continued to enlarge after 4 weeks, with subsequent development of necrotic tissue that required surgical excision. In the postmarketing period, additional cases of injection site reaction with features including induration, cellulitis, hematoma, abscess, sterile abscess, and necrosis, have been reported. Some cases required surgical intervention, including debridement of necrotic tissue. Some cases resulted in significant scarring. The reported cases occurred primarily in female patients.
VIVITROL is administered as a deep intramuscular gluteal injection, and inadvertent subcutaneous injection of VIVITROL may increase the likelihood of severe injection site reactions. The needles provided in the carton are customized needles. VIVITROL must not be injected using any other needle. The needle lengths (either 1 1/2 or 2 inches) may not be adequate in every patient because of body habitus. Body habitus should be assessed prior to each injection for each patient to assure that the proper needle is selected and that the needle length is adequate for intramuscular administration. For patients with a larger amount of subcutaneous tissue overlying the gluteal muscle, the administering healthcare provider may utilize the supplied 2-inch needle with needle protection device to help ensure that the injectate reaches the intramuscular mass. For very lean patients, the 1 1/2-inch needle may be appropriate to prevent the needle contacting the periosteum. Either needle may be used for patients with average body habitus. Healthcare providers should ensure that the VIVITROL injection is given correctly, and should consider alternate treatment for those patients whose body habitus precludes an intramuscular gluteal injection with one of the provided needles.
Patients should be informed that any concerning injection site reactions should be brought to the attention of the healthcare provider [see Patient Counseling Information (17 )] . Patients exhibiting signs of abscess, cellulitis, necrosis, or extensive swelling should be evaluated by a physician to determine if referral to a surgeon is warranted.
Precipitation of Opioid Withdrawal
The symptoms of spontaneous opioid withdrawal (which are associated with the discontinuation of opioid in a dependent individual) are uncomfortable, but they are not generally believed to be severe or necessitate hospitalization. However, when withdrawal is precipitated abruptly by the administration of an opioid antagonist to an opioid-dependent patient , the resulting withdrawal syndrome can be severe enough to require hospitalization. Review of postmarketing cases of precipitated opioid withdrawal in association with naltrexone treatment has identified cases with symptoms of withdrawal severe enough to require hospital admission, and in some cases, management in the intensive care unit.
To prevent occurrence of precipitated withdrawal in patients dependent on opioids, or exacerbation of a pre-existing subclinical withdrawal syndrome, opioid-dependent patients, including those being treated for alcohol dependence, should be opioid-free (including tramadol) before starting VIVITROL treatment. An opioid-free interval of a minimum of 7–10 days is recommended for patients previously dependent on short-acting opioids. Patients transitioning from buprenorphine or methadone may be vulnerable to precipitation of withdrawal symptoms for as long as two weeks.
If a more rapid transition from agonist to antagonist therapy is deemed necessary and appropriate by the healthcare provider, monitor the patient closely in an appropriate medical setting where precipitated withdrawal can be managed.
In every case, healthcare providers should always be prepared to manage withdrawal symptomatically with non-opioid medications because there is no completely reliable method for determining whether a patient has had an adequate opioid-free period. A naloxone challenge test may be helpful; however, a few case reports have indicated that patients may experience precipitated withdrawal despite having a negative urine toxicology screen or tolerating a naloxone challenge test (usually in the setting of transitioning from buprenorphine treatment). Patients should be made aware of the risks associated with precipitated withdrawal and encouraged to give an accurate account of last opioid use. Patients treated for alcohol dependence with VIVITROL should also be assessed for underlying opioid dependence and for any recent use of opioids prior to initiation of treatment with VIVITROL. Precipitated opioid withdrawal has been observed in alcohol-dependent patients in circumstances where the prescriber had been unaware of the additional use of opioids or co-dependence on opioids.
Hepatotoxicity
Cases of hepatitis and clinically significant liver dysfunction were observed in association with VIVITROL exposure during the clinical development program and in the postmarketing period. Transient, asymptomatic hepatic transaminase elevations were also observed in the clinical trials and postmarketing period. Although patients with clinically significant liver disease were not systematically studied, clinical trials did include patients with asymptomatic viral hepatitis infections. When patients presented with elevated transaminases, there were often other potential causative or contributory etiologies identified, including pre-existing alcoholic liver disease, hepatitis B and/or C infection, and concomitant usage of other potentially hepatotoxic drugs. Although clinically significant liver dysfunction is not typically recognized as a manifestation of opioid withdrawal, opioid withdrawal that is precipitated abruptly may lead to systemic sequelae including acute liver injury.
Patients should be warned of the risk of hepatic injury and advised to seek medical attention if they experience symptoms of acute hepatitis. Use of VIVITROL should be discontinued in the event of symptoms and/or signs of acute hepatitis.
Depression and Suicidality
Alcohol- and opioid-dependent patients, including those taking VIVITROL, should be monitored for the development of depression or suicidal thinking. Families and caregivers of patients being treated with VIVITROL should be alerted to the need to monitor patients for the emergence of symptoms of depression or suicidality, and to report such symptoms to the patient's healthcare provider.
Alcohol Dependence
In controlled clinical trials of VIVITROL administered to adults with alcohol dependence, adverse events of a suicidal nature (suicidal ideation, suicide attempts, completed suicides) were infrequent overall, but were more common in patients treated with VIVITROL than in patients treated with placebo (1% vs 0%). In some cases, the suicidal thoughts or behavior occurred after study discontinuation, but were in the context of an episode of depression that began while the patient was on study drug. Two completed suicides occurred, both involving patients treated with VIVITROL.
Depression-related events associated with premature discontinuation of study drug were also more common in patients treated with VIVITROL (~1%) than in placebo-treated patients (0%).
In the 24-week, placebo-controlled pivotal trial in 624 alcohol-dependent patients, adverse events involving depressed mood were reported by 10% of patients treated with VIVITROL 380 mg, as compared to 5% of patients treated with placebo injections.
Opioid Dependence
In an open-label, long-term safety study conducted in the US, adverse events of a suicidal nature (depressed mood, suicidal ideation, suicide attempt) were reported by 5% of opioid-dependent patients treated with VIVITROL 380 mg (n=101) and 10% of opioid-dependent patients treated with oral naltrexone (n=20). In the 24-week, placebo-controlled pivotal trial that was conducted in Russia in 250 opioid-dependent patients, adverse events involving depressed mood or suicidal thinking were not reported by any patient in either treatment group (VIVITROL 380 mg or placebo).
When Reversal of VIVITROL Blockade Is Required for Pain Management
In an emergency situation in patients receiving VIVITROL, suggestions for pain management include regional analgesia or use of non-opioid analgesics. If opioid therapy is required as part of anesthesia or analgesia, patients should be continuously monitored in an anesthesia care setting by persons not involved in the conduct of the surgical or diagnostic procedure. The opioid therapy must be provided by individuals specifically trained in the use of anesthetic drugs and the management of the respiratory effects of potent opioids, specifically the establishment and maintenance of a patent airway and assisted ventilation.
Irrespective of the drug chosen to reverse VIVITROL blockade, the patient should be monitored closely by appropriately trained personnel in a setting equipped and staffed for cardiopulmonary resuscitation.
Eosinophilic Pneumonia
In clinical trials with VIVITROL, there was one diagnosed case and one suspected case of eosinophilic pneumonia. Both cases required hospitalization, and resolved after treatment with antibiotics and corticosteroids. Similar cases have been reported in postmarketing use. Should a person receiving VIVITROL develop progressive dyspnea and hypoxemia, the diagnosis of eosinophilic pneumonia should be considered [see Adverse Reactions (6 )] . Patients should be warned of the risk of eosinophilic pneumonia, and advised to seek medical attention should they develop symptoms of pneumonia. Clinicians should consider the possibility of eosinophilic pneumonia in patients who do not respond to antibiotics.
Hypersensitivity Reactions Including Anaphylaxis
Cases of urticaria, angioedema, and anaphylaxis have been observed with use of VIVITROL in the clinical trial setting and in postmarketing use. Patients should be warned of the risk of hypersensitivity reactions, including anaphylaxis. In the event of a hypersensitivity reaction, patients should be advised to seek immediate medical attention in a healthcare setting prepared to treat anaphylaxis. The patient should not receive any further treatment with VIVITROL.
Intramuscular Injections
As with any intramuscular injection, VIVITROL should be administered with caution to patients with thrombocytopenia or any coagulation disorder (e.g., hemophilia and severe hepatic failure).
Alcohol Withdrawal
Use of VIVITROL does not eliminate nor diminish alcohol withdrawal symptoms.
Interference with Laboratory Tests
VIVITROL may be cross-reactive with certain immunoassay methods for the detection of drugs of abuse (specifically opioids) in urine. For further information, reference to the specific immunoassay instructions is recommended.
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Accidental Opioid Overdose [ see Warnings and Precautions (5.1 )]
- Injection Site Reactions [see Warnings and Precautions (5.2 )]
- Precipitated Opioid Withdrawal [see Warnings and Precautions (5.3 )]
- Hepatotoxicity [see Warnings and Precautions (5.4 )]
- Depression and Suicidality [see Warnings and Precautions (5.5 )]
- Eosinophilic Pneumonia [see Warnings and Precautions (5.7 )]
- Hypersensitivity Reactions [see Warnings and Precautions (5.8 )]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In all controlled and uncontrolled trials during the premarketing development of VIVITROL, more than 1100 patients with alcohol and/or opioid dependence have been treated with VIVITROL. Approximately 700 patients have been treated for 6 months or more, and more than 400 for 1 year or longer.
Adverse Events Leading to Discontinuation of Treatment
Alcohol Dependence
In controlled trials of 6 months or less in alcohol-dependent patients, 9% of alcohol-dependent patients treated with VIVITROL discontinued treatment due to an adverse event, as compared to 7% of the alcohol-dependent patients treated with placebo. Adverse events in the VIVITROL 380 mg group that led to more dropouts than in the placebo-treated group were injection site reactions (3%), nausea (2%), pregnancy (1%), headache (1%), and suicide-related events (0.3%). In the placebo group, 1% of patients withdrew due to injection site reactions, and 0% of patients withdrew due to the other adverse events.
Opioid Dependence
In a controlled trial of 6 months, 2% of opioid-dependent patients treated with VIVITROL discontinued treatment due to an adverse event, as compared to 2% of the opioid-dependent patients treated with placebo.
Common Adverse Reactions
Alcohol Dependence
Table 1 lists all treatment-emergent clinical adverse reactions, regardless of causality, occurring in ≥5% of patients with alcohol dependence, for which the incidence was greater in the combined VIVITROL group than in the placebo group. A majority of patients treated with VIVITROL in clinical studies had adverse reactions with a maximum intensity of “mild” or “moderate”.
| Body System | Adverse Reaction / Preferred Term | Placebo | Naltrexone for extended-release injectable suspension | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N=214 | 400 mg N=25 | 380 mg N=205 | 190 mg N=210 | All N=440 | |||||||
| N | % | N | % | N | % | N | % | N | % | ||
a) Includes the preferred terms: diarrhea NOS; frequent bowel movements; gastrointestinal upset; loose stools | |||||||||||
b) Includes the preferred terms: abdominal pain NOS; abdominal pain upper; stomach discomfort; abdominal pain lower | |||||||||||
c) Includes the preferred terms: nasopharyngitis; pharyngitis streptococcal; pharyngitis NOS | |||||||||||
d) Includes the preferred terms: anxiety NEC; anxiety aggravated; agitation; obsessive compulsive disorder; panic attack; nervousness; posttraumatic stress | |||||||||||
e) Includes the preferred terms: malaise; fatigue (these two comprise the majority of cases); lethargy; sluggishness | |||||||||||
f) Includes the preferred terms: muscle cramps; spasms; tightness; twitching; stiffness; rigidity | |||||||||||
g) Includes the preferred terms: rash NOS; rash papular; heat rash | |||||||||||
h) Includes the preferred terms: headache NOS; sinus headache; migraine; frequent headaches | |||||||||||
| Gastrointestinal Disorders | Nausea | 24 | 11 | 8 | 32 | 68 | 33 | 53 | 25 | 129 | 29 |
| Vomiting NOS | 12 | 6 | 3 | 12 | 28 | 14 | 22 | 10 | 53 | 12 | |
| Diarrhea a) | 21 | 10 | 3 | 12 | 27 | 13 | 27 | 13 | 57 | 13 | |
| Abdominal pain b) | 17 | 8 | 4 | 16 | 23 | 11 | 23 | 11 | 50 | 11 | |
| Dry Mouth | 9 | 4 | 6 | 24 | 10 | 5 | 8 | 4 | 24 | 5 | |
| Infections & Infestations | Pharyngitis c) | 23 | 11 | 0 | 0 | 22 | 11 | 35 | 17 | 57 | 13 |
| Psychiatric Disorders | Insomnia, sleep disorder | 25 | 12 | 2 | 8 | 29 | 14 | 27 | 13 | 58 | 13 |
| Anxiety d) | 17 | 8 | 2 | 8 | 24 | 12 | 16 | 8 | 42 | 10 | |
| Depression | 9 | 4 | 0 | 0 | 17 | 8 | 7 | 3 | 24 | 5 | |
| General Disorders & Administration Site Conditions | Any ISR | 106 | 50 | 22 | 88 | 142 | 69 | 121 | 58 | 285 | 65 |
| Injection site tenderness | 83 | 39 | 18 | 72 | 92 | 45 | 89 | 42 | 199 | 45 | |
| Injection site induration | 18 | 8 | 7 | 28 | 71 | 35 | 52 | 25 | 130 | 30 | |
| Injection site pain | 16 | 7 | 0 | 0 | 34 | 17 | 22 | 10 | 56 | 13 | |
| Other ISR (primarily nodules, swelling) | 8 | 4 | 8 | 32 | 30 | 15 | 16 | 8 | 54 | 12 | |
| Injection site pruritus | 0 | 0 | 0 | 0 | 21 | 10 | 13 | 6 | 34 | 8 | |
| Injection site ecchymosis | 11 | 5 | 0 | 0 | 14 | 7 | 9 | 4 | 23 | 5 | |
| Asthenic conditions e) | 26 | 12 | 3 | 12 | 47 | 23 | 40 | 19 | 90 | 20 | |
| Musculoskeletal & Connective Tissue Disorders | Arthralgia, arthritis, joint stiffness | 11 | 5 | 1 | 4 | 24 | 12 | 12 | 6 | 37 | 9 |
| Back pain, back stiffness | 10 | 5 | 1 | 4 | 12 | 6 | 14 | 7 | 27 | 6 | |
| Muscle cramps f) | 3 | 1 | 0 | 0 | 16 | 8 | 5 | 2 | 21 | 5 | |
| Skin & Subcutaneous Tissue Disorders | Rash g) | 8 | 4 | 3 | 12 | 12 | 6 | 10 | 5 | 25 | 6 |
| Nervous System Disorders | Headache h) | 39 | 18 | 9 | 36 | 51 | 25 | 34 | 16 | 94 | 21 |
| Dizziness, syncope | 9 | 4 | 4 | 16 | 27 | 13 | 27 | 13 | 58 | 13 | |
| Somnolence, sedation | 2 | 1 | 3 | 12 | 8 | 4 | 9 | 4 | 20 | 5 | |
| Metabolism & Nutrition Disorders | Anorexia, appetite decreased NOS, appetite disorder NOS | 6 | 3 | 5 | 20 | 30 | 14 | 13 | 6 | 48 | 11 |
Opioid Dependence
In the open-label, long-term safety study conducted in the US, the commonly reported adverse reactions among the opioid-dependent patients in the study were similar to those commonly observed events in the alcohol-dependent populations in VIVITROL clinical trials as displayed in Table 1 , above. For example, injection site reactions of all types, nausea and diarrhea occurred in more than 5% of patients on VIVITROL in the open-label study. In contrast, 48% percent, of the opioid-dependent patients had at least one adverse event in the “Infections and Infestations” Body System. Adverse Reactions/Preferred Terms of nasopharyngitis, upper respiratory tract infection, urinary tract infection, and sinusitis were most commonly reported.
In the placebo-controlled study in opioid-dependent patients conducted in Russia, the overall frequency of adverse events was lower than in the U.S. population described above. Table 2 lists treatment-emergent clinical adverse events, regardless of causality, occurring in ≥2% of patients with opioid dependence, for which the incidence was greater in the VIVITROL group than in the placebo group. All adverse events were assessed as having a maximum intensity of “mild” or “moderate.”
| Body System | Adverse Event / Preferred Term | Placebo N=124 | VIVITROL 380 mg N=126 | ||
| n | % | n | % | ||
| Investigations | Alanine aminotransferase increased | 7 | 6 | 16 | 13 |
| Aspartate aminotransferase increased | 3 | 2 | 13 | 10 | |
| Gamma-glutamyltransferase increased | 4 | 3 | 9 | 7 | |
| Infections and Infestations | Nasopharyngitis | 3 | 2 | 9 | 7 |
| Influenza | 5 | 4 | 6 | 5 | |
| Psychiatric Disorders | Insomnia | 1 | 1 | 8 | 6 |
| Vascular Disorders | Hypertension | 4 | 3 | 6 | 5 |
| General Disorders and Administration Site Conditions | Injection site pain | 1 | 1 | 6 | 5 |
| Gastrointestinal Disorders | Toothache | 2 | 2 | 5 | 4 |
| Nervous System Disorders | Headache | 3 | 2 | 4 | 3 |
Laboratory Tests
Eosinophil Count
In clinical trials, subjects on VIVITROL had increases in eosinophil counts relative to subjects on placebo. With continued use of VIVITROL, eosinophil counts returned to normal over a period of several months.
Platelet Count
VIVITROL 380 mg was associated with a decrease in platelet count. In clinical trials, alcohol-dependent patients treated with VIVITROL experienced a mean maximal decrease in platelet count of 17.8 × 10 3 /μL, compared to 2.6 × 10 3 /μL in placebo patients.
After 24 weeks of treatment, opioid-dependent patients treated with VIVITROL experienced a mean maximal decrease in platelet count of 62.8 × 10 3 /μL, compared to 39.9 × 10 3 /μL in placebo patients. In randomized controlled trials, VIVITROL was not associated with an increase in bleeding-related adverse events.
Hepatic Enzyme Elevations
In short-term, controlled trials, in alcohol-dependent patients, the incidence of AST elevations associated with VIVITROL treatment was similar to that observed with oral naltrexone treatment (1.5% each) and slightly higher than observed with placebo treatment (0.9%).
In the 6-month controlled trial conducted in opioid-dependent subjects, 89% had a baseline diagnosis of hepatitis C infection, and 41% had a baseline diagnosis of HIV infection. There were frequently observed elevated liver enzyme levels (ALT, AST, and GGT); these were more commonly reported as adverse events in the VIVITROL 380 mg group than in the placebo group. Patients could not enroll in this trial if they had a baseline ALT or AST value that was more than three times the upper limit of normal. More patients treated with VIVITROL in this study experienced treatment-emergent elevations in transaminases to more than three times the upper limit of normal than patients treated with placebo. Shifts to more than three times the upper limit of normal occurred in 20% of patients treated with VIVITROL as compared with 13% of placebo patients. Shifts in values of AST to more than three times the upper limit were also more common in the VIVITROL (14%) arm compared with the placebo (11%) arm. Opioid-dependent patients treated with VIVITROL experienced a mean maximal increase from baseline ALT levels of 61 IU/L compared with 48 IU/L in placebo patients. Similarly for AST, opioid-dependent patients treated with VIVITROL experienced a mean maximal increase from baseline AST levels of 40 IU/L compared with 31 IU/L in placebo patients.
Creatinine Phosphokinase
In short-term controlled trials in alcohol-dependent patients, more patients treated with VIVITROL 380 mg (11%) and oral naltrexone (17%) shifted from normal creatinine phosphokinase (CPK) levels before treatment to abnormal CPK levels at the end of the trials, compared to placebo patients (8%). In open-label trials, 16% of patients dosed for more than 6 months had increases in CPK. For both the oral naltrexone and VIVITROL 380 mg groups, CPK abnormalities were most frequently in the range of 1–2 × ULN. However, there were reports of CPK abnormalities as high as 4x ULN for the oral naltrexone group, and 35 × ULN for the VIVITROL 380 mg group. Overall, there were no differences between the placebo and naltrexone (oral or injectable) groups with respect to the proportions of patients with a CPK value at least three times the upper limit of normal. No factors other than naltrexone exposure were associated with the CPK elevations.
More opioid-dependent patients treated with VIVITROL 380 mg (39%) shifted from normal creatinine phosphokinase (CPK) levels before treatment to abnormal CPK levels during the study as compared to patients treated with placebo (32%). There were reports of CPK abnormalities as high as 41.8 × ULN for the placebo group, and 22.1 × ULN for the VIVITROL 380 mg group.
Other Events Observed During the VIVITROL Clinical Studies
The following is a list of treatment-emergent adverse reactions reported by alcohol- and/or opioid-dependent subjects treated with VIVITROL in all clinical trials. The listing does not include those events already listed in the previous tables or elsewhere in labeling, those events for which a drug cause was remote, those events that were so general as to be uninformative, and those events reported only once that did not have a substantial probability of being acutely life-threatening.
Blood and Lymphatic System Disorders – lymphadenopathy (including cervical adenitis), white blood cell count increased
Cardiac Disorders – angina pectoris, angina unstable, atrial fibrillation, cardiac failure congestive, coronary artery atherosclerosis, myocardial infarction, palpitations
Eye Disorders – conjunctivitis, vision blurred
Gastrointestinal Disorders – abdominal discomfort, colitis, constipation, flatulence, gastroesophageal reflux disease, gastrointestinal hemorrhage, hemorrhoids, pancreatitis acute, paralytic ileus, perirectal abscess
General Disorders and Administration Site Conditions – chest pain, chest tightness, chills, face edema, irritability, lethargy, pyrexia, rigors
Hepatobiliary Disorders – cholecystitis acute, cholelithiasis
Immune System Disorders – seasonal allergy, hypersensitivity reaction (including angioneurotic edema and urticaria)
Infections and Infestations – bronchitis, gastroenteritis, laryngitis, pneumonia, sinusitis, tooth abscess, upper respiratory tract infection, urinary tract infection, advanced HIV disease in HIV-infected patients
Investigations – weight decreased, weight increased
Metabolism and Nutrition Disorders – appetite increased, dehydration, heat exhaustion, hypercholesterolemia
Musculoskeletal and Connective Tissue Disorders – joint stiffness, muscle spasms, myalgia, pain in limb
Nervous System Disorders – cerebral arterial aneurysm, convulsions, disturbance in attention, dysgeusia, mental impairment, migraine, ischemic stroke, paresthesia
Pregnancy, Puerperium, and Perinatal Conditions – abortion missed
Psychiatric Disorders – abnormal dreams, agitation, alcohol withdrawal syndrome, euphoric mood, delirium, libido decreased
Respiratory, Thoracic, and Mediastinal Disorders – chronic obstructive pulmonary disease, dyspnea, pharyngolaryngeal pain, sinus congestion
Skin and Subcutaneous Tissue Disorders – night sweats, pruritus, sweating increased
Vascular Disorders – deep venous thrombosis, hot flushes, pulmonary embolism
Postmarketing Experience
Adverse Events Following Patient Self-Administration
Adverse events including injection site reactions and precipitated opioid withdrawal resulting in serious outcomes, including hospitalization, have been reported following patient self-administration of VIVITROL. VIVITROL must be prepared and administered by a healthcare provider.
Hypersensitivity Reactions Including Anaphylaxis
Hypersensitivity reactions including anaphylaxis have been reported during postmarketing surveillance.
Reports From Other Intramuscular Drug Products Containing Polylactide-co-glycolide (PLG) Microspheres
Retinal Artery Occlusion
Retinal artery occlusion after injection with another drug product containing polylactide-co-glycolide (PLG) microspheres has been reported very rarely during postmarketing surveillance. This event has been reported in the presence of abnormal arteriovenous anastomosis. No cases of retinal artery occlusion have been reported during VIVITROL clinical trials or postmarketing surveillance. VIVITROL should be administered by intramuscular (IM) injection into the gluteal muscle, and care must be taken to avoid inadvertent injection into a blood vessel [see Dosage and Administration (2 )] .
DRUG INTERACTIONS
Patients taking VIVITROL may not benefit from opioid-containing medicines. Naltrexone antagonizes the effects of opioid-containing medicines, such as cough and cold remedies, antidiarrheal preparations and opioid analgesics.
DESCRIPTION
VIVITROL ® (naltrexone for extended-release injectable suspension) is supplied as a microsphere formulation of naltrexone for suspension, to be administered by deep intramuscular injection by a healthcare practitioner. VIVITROL contains naltrexone base anhydrous as the active pharmaceutical ingredient. Naltrexone is an opioid antagonist with little, if any, opioid agonist activity.
Naltrexone is morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-(5α) (CAS Registry # 16590-41-3). The molecular formula is C 20 H 23 NO 4 and its molecular weight is 341.41 in the anhydrous form (ie, < 1% maximum water content). The structural formula is:
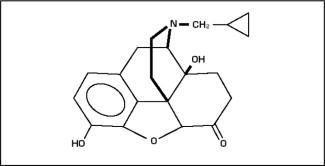
Naltrexone base anhydrous is an off-white to a light tan powder with a melting point of 168 °C to 170 °C (334 °F to 338 °F). It is insoluble in water and is soluble in ethanol.
VIVITROL is provided in a carton containing one vial of VIVITROL microspheres, one vial of diluent, one 5-mL syringe, one 1-inch 20-gauge preparation needle, two 1 1/2-inch 20-gauge, and two 2-inch 20-gauge administration needles with needle protection device.
VIVITROL microspheres consist of a sterile, off-white to light tan powder that is available in a dosage strength of 380 mg of naltrexone per vial. Naltrexone base anhydrous is incorporated in a biodegradable matrix of 75:25 polylactide-co-glycolide (PLG) at a concentration of 337 mg of naltrexone per gram of microspheres.
The diluent is a clear, colorless solution. Each mL of diluent contains 30.0 mg of carboxymethylcellulose sodium, 1.0 mg of polysorbate 20, 9.0 mg of sodium chloride, and sodium hydroxide and hydrochloric acid as pH adjusters, in water for injection. The pH of the diluent is in the range of 5.8 to 7.2.
CLINICAL PHARMACOLOGY
Mechanism of Action
Naltrexone is an opioid antagonist with highest affinity for the mu opioid receptor. Naltrexone has little or no opioid agonist activity.
Pharmacodynamics
Naltrexone has few, if any, intrinsic actions besides its opioid blocking properties. However, it does produce some pupillary constriction, by an unknown mechanism.
The administration of VIVITROL is not associated with the development of tolerance or dependence. In subjects physically dependent on opioids, VIVITROL will precipitate withdrawal symptomatology.
Occupation of opioid receptors by naltrexone may block the effects of endogenous opioid peptides. It markedly attenuates or completely blocks, reversibly, the subjective effects of exogenous opioids. The neurobiological mechanisms responsible for the reduction in alcohol consumption observed in alcohol-dependent patients treated with naltrexone are not entirely understood. However, involvement of the endogenous opioid system is suggested by preclinical data.
Naltrexone blocks the effects of opioids by competitive binding at opioid receptors. This makes the blockade produced potentially surmountable, but overcoming full naltrexone blockade by administration of opioids may result in non-opioid receptor-mediated symptoms such as histamine release.
VIVITROL is not aversive therapy and does not cause a disulfiram-like reaction either as a result of opioid use or ethanol ingestion.
Pharmacokinetics
Absorption
VIVITROL is an extended-release, microsphere formulation of naltrexone designed to be administered by intramuscular (IM) gluteal injection every 4 weeks or once a month. After IM injection, the naltrexone plasma concentration time profile is characterized by a transient initial peak, which occurs approximately 2 hours after injection, followed by a second peak observed approximately 2-3 days later. Beginning approximately 14 days after dosing, concentrations slowly decline, with measurable levels for greater than 1 month.
Maximum plasma concentration (C max ) and area under the curve (AUC) for naltrexone and 6β-naltrexol (the major metabolite) following VIVITROL administration are dose proportional. Compared to daily oral dosing with naltrexone 50 mg over 28 days, total naltrexone exposure is 3 to 4-fold higher following administration of a single dose of VIVITROL 380 mg. Steady state is reached at the end of the dosing interval following the first injection. There is minimal accumulation (<15%) of naltrexone or 6β-naltrexol upon repeat administration of VIVITROL.
Distribution
In vitro data demonstrate that naltrexone plasma protein binding is low (21%).
Elimination
The elimination half life of naltrexone following VIVITROL administration is 5-10 days and is dependent on the erosion of the polymer. The elimination half life of 6β-naltrexol following VIVITROL administration is 5-10 days.
Metabolism
Naltrexone is extensively metabolized in humans. Production of the primary metabolite, 6β-naltrexol, is mediated by dihydrodiol dehydrogenase, a cytosolic family of enzymes. The cytochrome P450 system is not involved in naltrexone metabolism. Two other minor metabolites are 2-hydroxy-3-methoxy-6β-naltrexol and 2-hydroxy-3-methoxy-naltrexone. Naltrexone and its metabolites are also conjugated to form glucuronide products.
Significantly less 6β-naltrexol is generated following IM administration of VIVITROL compared to administration of oral naltrexone due to a reduction in first-pass hepatic metabolism.
Excretion
Elimination of naltrexone and its metabolites occurs primarily via urine, with minimal excretion of unchanged naltrexone.
Specific Populations
Pediatric: Pharmacokinetics of VIVITROL have not been evaluated in a pediatric population.
Geriatric: Pharmacokinetics of VIVITROL have not been evaluated in the geriatric population [see Use in Specific Populations (8.5 )] .
Race: Effect of race on the pharmacokinetics of VIVITROL has not been studied.
Sex: In a study in healthy subjects (n=18 females and 18 males), sex did not influence the pharmacokinetics of VIVITROL.
Renal Insufficiency: A population pharmacokinetic analysis indicated mild renal insufficiency (creatinine clearance of 50-80 mL/min) had little or no influence on VIVITROL pharmacokinetics and that no dosage adjustment is necessary. VIVITROL pharmacokinetics have not been evaluated in subjects with moderate and severe renal insufficiency [see Use in Specific Populations (8.6 )] .
Hepatic Insufficiency: The pharmacokinetics of VIVITROL are not altered in subjects with mild to moderate hepatic impairment (Groups A and B of the Child-Pugh classification). VIVITROL pharmacokinetics were not evaluated in subjects with severe hepatic impairment [see Use in Specific Populations (8.7 )] .
Drug Interactions
In Vitro Studies: Because naltrexone is not a substrate for CYP drug metabolizing enzymes, inducers or inhibitors of these enzymes are unlikely to change the clearance of VIVITROL. An in vitro CYP inhibition study demonstrated that naltrexone is not an inhibitor of major CYP enzymes (CYP 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4). An in vitro CYP induction study demonstrated that naltrexone is not an inducer of CYP3A4 and CYP1A2.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Carcinogenicity studies have not been conducted with VIVITROL.
Carcinogenicity studies of oral naltrexone hydrochloride (administered via the diet) have been conducted in rats and mice.
In a two-year carcinogenicity study in rats, there were small increases in the numbers of testicular mesotheliomas in males and tumors of vascular origin in males and females. The incidence of testicular mesothelioma in males given naltrexone at a dietary dose of 100 mg/kg/day (3-times the human exposure based on an AUC (0-28d) comparison) was 6%, compared with a maximum historical incidence of 4%. The incidence of vascular tumors in males and females given dietary doses of 100 mg/kg/day was 4% but only the incidence in females was increased compared with a maximum historical control incidence of 2% (3 and 32 times the human exposure based on an AUC (0-28d) comparison in males and females, respectively). There was no evidence of carcinogenicity in a 2-year dietary study with naltrexone in male and female mice (12 and 3 times the human exposure based on an AUC (0-28d) comparison, respectively). The clinical significance of these findings is not known.
Mutagenesis: Naltrexone was negative in the following in vitro genotoxicity studies: bacterial reverse mutation assay (Ames test), the heritable translocation assay, CHO cell sister chromatid exchange assay, and the mouse lymphoma gene mutation assay. Naltrexone was also negative in an in vivo mouse micronucleus assay. In contrast, naltrexone tested positive in the following assays: Drosophila recessive lethal frequency assay, non-specific DNA damage in repair tests with E. coli and WI-38 cells, and urinalysis for methylated histidine residues.
Impairment of Fertility: Daily oral administration of naltrexone caused a significant increase in pseudopregnancy and a decrease in pregnancy rates in rats at 100 mg/kg/day (75 times the human exposure based on an AUC (0-28d) comparison). There was no effect on male fertility at this dose level (6 times the human exposure based on an AUC (0-28d) comparison). The relevance of these observations to human fertility is not known.
CLINICAL STUDIES
Alcohol Dependence
The efficacy of VIVITROL in the treatment of alcohol dependence was evaluated in a 24-week, placebo-controlled, multi-center, double-blind, randomized trial of alcohol-dependent (DSM-IV criteria) outpatients. Subjects were treated with an injection every 4 weeks of VIVITROL 190 mg, VIVITROL 380 mg or placebo. Oral naltrexone was not administered prior to the initial or subsequent injections of study medication. Psychosocial support was provided to all subjects in addition to medication.
Subjects treated with VIVITROL 380 mg demonstrated a greater reduction in days of heavy drinking than those treated with placebo. Heavy drinking was defined as self-report of 5 or more standard drinks consumed on a given day for male patients and 4 or more drinks for female patients. Among the subset of patients (n=53, 8% of the total study population) who abstained completely from drinking during the week prior to the first dose of medication, compared with placebo-treated patients, those treated with VIVITROL 380 mg had greater reductions in the number of drinking days and the number of heavy drinking days. In this subset, patients treated with VIVITROL were also more likely than placebo-treated patients to maintain complete abstinence throughout treatment. The same treatment effects were not evident among the subset of patients (n=571, 92% of the total study population) who were actively drinking at the time of treatment initiation.
Opioid Dependence
The efficacy of VIVITROL in the treatment of opioid dependence was evaluated in a 24-week, placebo-controlled, multi-center, double-blind, randomized trial of opioid-dependent (DSM-IV) outpatients, who were completing or had recently completed detoxification. Subjects were treated with an injection every 4 weeks of VIVITROL 380 mg or placebo. Oral naltrexone was not administered prior to the initial or subsequent injections of study medication. Standardized, manual-based psychosocial support was provided on a biweekly basis to all subjects in addition to medication.
Figure 1 , below, displays the cumulative percentage of subjects with opioid-free weeks ranging from no visits (0%) to all visits (100%). An opioid-free week was one in which urine drug test results were negative for opioids and self-reported opioid use was also zero. An initial period of engagement in treatment was permitted during which opiate use, if it occurred, was not considered in the analysis. Subjects discontinuing from the trial were assumed to have had opioid-use weeks for the weeks after dropout.
The cumulative percentage of subjects achieving each observed percentage of opioid-free weeks was greater in the VIVITROL group compared to the placebo group. Complete abstinence (opioid-free at all weekly visits) was sustained by 23% of subjects in the placebo group compared with 36% of subjects in the VIVITROL group from Week 5 to Week 24.
Figure 1: Subjects Sustaining Varying Percentages of Opioid-Free Weeks
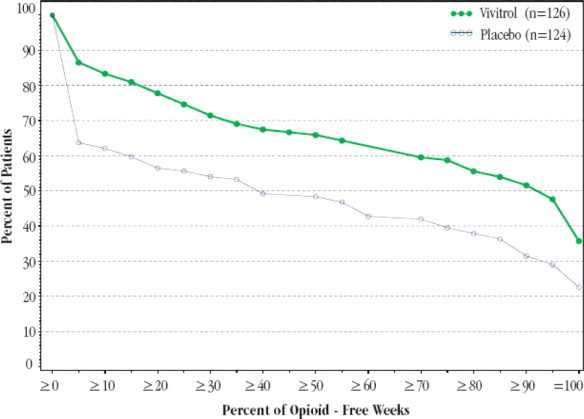
A greater percentage of subjects in the VIVITROL group remained in the study compared to the placebo group.
HOW SUPPLIED/STORAGE AND HANDLING
VIVITROL (naltrexone for extended-release injectable suspension) is supplied in cartons (NDC 65757-300-01). Each carton contains:
- one 380 mg vial of VIVITROL microspheres in a 5 mL single-dose vial,
- one vial containing 4 mL of diluent (to deliver 3.4 mL) for the suspension of VIVITROL,
- one 5-mL prepackaged syringe,
- one 20-gauge 1-inch needle,
- two 20-gauge 1 1/2-inch needles with needle protection devices, and;
- two 20-gauge 2-inch needles with needle protection devices
VIVITROL must be prepared and administered by a healthcare provider.
Storage and Handling
The entire dose pack should be stored in the refrigerator (2 °C to 8 °C, 36 °F to 46 °F). Unrefrigerated, VIVITROL can be stored at temperatures not exceeding 25 °C (77 °F) for no more than 7 days prior to administration. Do not expose the product to temperatures above 25 °C (77 °F). VIVITROL should not be frozen.
Keep out of Reach of Children.
Mechanism of Action
Naltrexone is an opioid antagonist with highest affinity for the mu opioid receptor. Naltrexone has little or no opioid agonist activity.