Xacduro patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- Administer XACDURO (1 g of sulbactam, 1 g of durlobactam) every 6 hours by intravenous (IV) infusion over 3 hours in patients with creatinine clearance (CLcr) of 45 to 129 mL/min. (2.1 )
- Dosing regimen adjustments are recommended for CLcr less than 45 mL/min and CLcr greater than or equal to 130 mL/min. (2.2 )
- Administer all doses of XACDURO by IV infusion over 3 hours. (2.3 )
- See full prescribing information for instructions on the preparation of the intravenous solution. (2.4 )
Recommended Dosage
XACDURO is a co-packaged product containing sulbactam for injection and durlobactam for injection. The recommended dosage of XACDURO is 1 gram (g) of sulbactam and 1 g of durlobactam every 6 hours administered by intravenous (IV) infusion over 3 hours in adults with a creatinine clearance (CLcr) of 45 to 129 mL/min.
Adjustments to the dosing regimen for XACDURO are recommended for patients with CLcr less than 45 mL/min and for patients with CLcr greater than or equal to 130 mL/min [see Dosage and Administration (2.2) ] .
The recommended duration of treatment with XACDURO is 7 to 14 days. The duration of therapy should be guided by the patient's clinical status.
Dosage in Patients (18 Years of Age and Older) Based on Renal Function
See Table 1 for the recommended dosage of XACDURO in patients (18 years of age and older) based on renal function [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3) ] .
Adjustments to the dosing regimen are recommended for patients with creatinine clearance (CLcr) less than 45 mL/min and patients with augmented renal clearance (CLcr greater than or equal to 130 mL/min). For patients undergoing intermittent hemodialysis (HD), start the dosing of XACDURO immediately after the completion of HD.
For patients with fluctuating renal function, monitor CLcr and adjust dosage accordingly [see Use in Specific Populations (8.6) ] .
| Dose of Sulbactam and Durlobactam (g) | Estimated CLcr (mL/min) CLcr = creatinine clearance estimated by Cockcroft-Gault equation | Frequency |
|---|---|---|
| sulbactam 1 g and durlobactam 1 g | Greater than or equal to 130 | Every 4 hours |
| 45 to 129 | Every 6 hours | |
| 30 to 44 | Every 8 hours | |
| 15 to 29 | Every 12 hours | |
| less than 15 For patients on hemodialysis, the dose should be administered after the dialysis session has ended | For patients initiating XACDURO: Every 12 hours for the first 3 doses (0, 12, and 24 hours), followed by every 24 hours after the third dose For patients currently receiving XACDURO whose CLcr declines to less than 15 mL/min: Every 24 hours |
Administration
The prepared XACDURO solution [see Dosage and Administration (2.4) ] should be brought to ambient room temperature (over 15 to 30 min) prior to infusion to the patient. Administer all doses of XACDURO by intravenous (IV) infusion over 3 hours.
Preparation of XACDURO for Intravenous Administration
XACDURO is a co-packaged kit containing 1 clear single-dose vial of sulbactam 1g and 2 amber single-dose vials of durlobactam 0.5g as sterile powders that must be reconstituted and further diluted using aseptic technique prior to intravenous infusion.
XACDURO does not contain a bacteriostatic preservative and the prepared solution must be used within 24 hours when stored refrigerated at 2°C to 8°C (36°F to 46°F). Discard unused portion.
Items required to prepare XACDURO:
- XACDURO kit (includes one sulbactam 1 g single-dose vial and two durlobactam 0.5 g single-dose vials)
- 100 mL infusion bag containing 0.9% Sodium Chloride Injection, USP
- 10 mL Sterile Water for Injection, USP
- 10 mL sterile syringe and alcohol wipes
Preparation of XACDURO:
- Reconstitute the sulbactam 1 g single-dose vial with 5 mL of Sterile Water for Injection and gently shake to dissolve. Each reconstituted vial contains 1 g of sulbactam per 5 mL of clear, colorless to slightly yellow solution. The reconstituted solution is not for direct injection and must be diluted before intravenous infusion. Dilution must occur within 1 hour of reconstitution.
- Reconstitute each durlobactam 0.5 g single-dose vial with 2.5 mL of Sterile Water for Injection and gently shake to dissolve. Each reconstituted vial contains 0.5 g of durlobactam per 2.5 mL of clear, light yellow to orange solution. The reconstituted solution is not for direct injection and must be diluted before intravenous infusion. Dilution must occur within 1 hour of reconstitution.
- To prepare the required XACDURO dose, withdraw 5 mL of reconstituted sulbactam and 5 mL (2.5 mL from each vial) of reconstituted durlobactam. Add the withdrawn volume of both sulbactam and durlobactam to a 100 mL infusion bag of 0.9% Sodium Chloride for Injection, USP. Discard unused portion.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The prepared XACDURO solution should appear as a clear, light yellow to orange solution, free of particulates. If the XACDURO solution is cloudy or contains particulates, do not administer.
Compatibility
XACDURO is compatible with 0.9% Sodium Chloride Injection, USP.
The compatibility of XACDURO for administration with solutions containing other drugs or other diluents has not been established. XACDURO should not be mixed with other drugs or physically added to solutions containing other drugs.
Storage of Prepared Solution in Infusion Bag
Store the prepared bag in the refrigerator at 2°C to 8°C (36°F to 46°F) until administration. The time between starting the reconstitution of the powders and the conclusion of the infusion should not exceed 24 hours. Do not freeze.

By using PrescriberAI, you agree to the AI Terms of Use.
Xacduro prescribing information
INDICATIONS AND USAGE
XACDURO is a co-packaged product containing sulbactam, a beta-lactam antibacterial and beta lactamase inhibitor, and durlobactam, a beta lactamase inhibitor, indicated in patients 18 years of age and older for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP), caused by susceptible isolates of Acinetobacter baumannii-calcoaceticus complex. (1.1 )
Limitations of Use :
XACDURO is not indicated for the treatment of HABP/VABP caused by pathogens other than susceptible isolates of Acinetobacter baumannii-calcoaceticus complex. (1.1 , 14 )
To reduce the development of drug-resistant bacteria and maintain the effectiveness of XACDURO and other antibacterial drugs, XACDURO should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.2 )
Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP)
XACDURO is indicated in patients 18 years of age and older for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP), caused by susceptible isolates of Acinetobacter baumannii-calcoaceticus complex.
Limitations of Use
XACDURO is not indicated for the treatment of HABP/VABP caused by pathogens other than susceptible isolates of Acinetobacter baumannii-calcoaceticus complex [see Clinical Studies (14) ] .
Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of XACDURO and other antibacterial drugs, XACDURO should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
DOSAGE AND ADMINISTRATION
- Administer XACDURO (1 g of sulbactam, 1 g of durlobactam) every 6 hours by intravenous (IV) infusion over 3 hours in patients with creatinine clearance (CLcr) of 45 to 129 mL/min. (2.1 )
- Dosing regimen adjustments are recommended for CLcr less than 45 mL/min and CLcr greater than or equal to 130 mL/min. (2.2 )
- Administer all doses of XACDURO by IV infusion over 3 hours. (2.3 )
- See full prescribing information for instructions on the preparation of the intravenous solution. (2.4 )
Recommended Dosage
XACDURO is a co-packaged product containing sulbactam for injection and durlobactam for injection. The recommended dosage of XACDURO is 1 gram (g) of sulbactam and 1 g of durlobactam every 6 hours administered by intravenous (IV) infusion over 3 hours in adults with a creatinine clearance (CLcr) of 45 to 129 mL/min.
Adjustments to the dosing regimen for XACDURO are recommended for patients with CLcr less than 45 mL/min and for patients with CLcr greater than or equal to 130 mL/min [see Dosage and Administration (2.2) ] .
The recommended duration of treatment with XACDURO is 7 to 14 days. The duration of therapy should be guided by the patient's clinical status.
Dosage in Patients (18 Years of Age and Older) Based on Renal Function
See Table 1 for the recommended dosage of XACDURO in patients (18 years of age and older) based on renal function [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3) ] .
Adjustments to the dosing regimen are recommended for patients with creatinine clearance (CLcr) less than 45 mL/min and patients with augmented renal clearance (CLcr greater than or equal to 130 mL/min). For patients undergoing intermittent hemodialysis (HD), start the dosing of XACDURO immediately after the completion of HD.
For patients with fluctuating renal function, monitor CLcr and adjust dosage accordingly [see Use in Specific Populations (8.6) ] .
| Dose of Sulbactam and Durlobactam (g) | Estimated CLcr (mL/min) CLcr = creatinine clearance estimated by Cockcroft-Gault equation | Frequency |
|---|---|---|
| sulbactam 1 g and durlobactam 1 g | Greater than or equal to 130 | Every 4 hours |
| 45 to 129 | Every 6 hours | |
| 30 to 44 | Every 8 hours | |
| 15 to 29 | Every 12 hours | |
| less than 15 For patients on hemodialysis, the dose should be administered after the dialysis session has ended | For patients initiating XACDURO: Every 12 hours for the first 3 doses (0, 12, and 24 hours), followed by every 24 hours after the third dose For patients currently receiving XACDURO whose CLcr declines to less than 15 mL/min: Every 24 hours |
Administration
The prepared XACDURO solution [see Dosage and Administration (2.4) ] should be brought to ambient room temperature (over 15 to 30 min) prior to infusion to the patient. Administer all doses of XACDURO by intravenous (IV) infusion over 3 hours.
Preparation of XACDURO for Intravenous Administration
XACDURO is a co-packaged kit containing 1 clear single-dose vial of sulbactam 1g and 2 amber single-dose vials of durlobactam 0.5g as sterile powders that must be reconstituted and further diluted using aseptic technique prior to intravenous infusion.
XACDURO does not contain a bacteriostatic preservative and the prepared solution must be used within 24 hours when stored refrigerated at 2°C to 8°C (36°F to 46°F). Discard unused portion.
Items required to prepare XACDURO:
- XACDURO kit (includes one sulbactam 1 g single-dose vial and two durlobactam 0.5 g single-dose vials)
- 100 mL infusion bag containing 0.9% Sodium Chloride Injection, USP
- 10 mL Sterile Water for Injection, USP
- 10 mL sterile syringe and alcohol wipes
Preparation of XACDURO:
- Reconstitute the sulbactam 1 g single-dose vial with 5 mL of Sterile Water for Injection and gently shake to dissolve. Each reconstituted vial contains 1 g of sulbactam per 5 mL of clear, colorless to slightly yellow solution. The reconstituted solution is not for direct injection and must be diluted before intravenous infusion. Dilution must occur within 1 hour of reconstitution.
- Reconstitute each durlobactam 0.5 g single-dose vial with 2.5 mL of Sterile Water for Injection and gently shake to dissolve. Each reconstituted vial contains 0.5 g of durlobactam per 2.5 mL of clear, light yellow to orange solution. The reconstituted solution is not for direct injection and must be diluted before intravenous infusion. Dilution must occur within 1 hour of reconstitution.
- To prepare the required XACDURO dose, withdraw 5 mL of reconstituted sulbactam and 5 mL (2.5 mL from each vial) of reconstituted durlobactam. Add the withdrawn volume of both sulbactam and durlobactam to a 100 mL infusion bag of 0.9% Sodium Chloride for Injection, USP. Discard unused portion.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The prepared XACDURO solution should appear as a clear, light yellow to orange solution, free of particulates. If the XACDURO solution is cloudy or contains particulates, do not administer.
Compatibility
XACDURO is compatible with 0.9% Sodium Chloride Injection, USP.
The compatibility of XACDURO for administration with solutions containing other drugs or other diluents has not been established. XACDURO should not be mixed with other drugs or physically added to solutions containing other drugs.
Storage of Prepared Solution in Infusion Bag
Store the prepared bag in the refrigerator at 2°C to 8°C (36°F to 46°F) until administration. The time between starting the reconstitution of the powders and the conclusion of the infusion should not exceed 24 hours. Do not freeze.
DOSAGE FORMS AND STRENGTHS
XACDURO is a co-packaged kit containing the following two components as sterile powders for reconstitution:
- 1 clear single-dose vial of sulbactam for injection 1g (as white to off-white powder) and
- 2 amber single-dose vials of durlobactam for injection 0.5g (as solid cake or powder) in each vial.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
XACDURO
There are no available data on the use of XACDURO in pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes.
Individual Components of XACDURO
Sulbactam:
Available published data from case reports and case series with sulbactam use in combination with ampicillin during pregnancy over many decades have not identified a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. The published literature reports that sulbactam crosses the human placenta. Reproduction studies have been performed in mice, rats, and rabbits at doses up to ten (10) times the human dose and have revealed no evidence of harm to the fetus due to sulbactam (see Data ).
Durlobactam:
There are no available data on the use of durlobactam in pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes.
Durlobactam administered to pregnant mice and rats during organogenesis, showed no drug-induced fetal malformations but an increased incidence of skeletal variations was observed in mice at 2- and 4-times the Maximum Recommended Human Dose (MRHD) (see Data ) .
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Sulbactam:
Reproduction studies have been performed in mice, rats, and rabbits at doses up to ten (10) times the human dose and have revealed no evidence of harm to the fetus due to sulbactam sodium/ampicillin sodium.
Durlobactam:
Daily administration of durlobactam at 400, 800, or 1600 mg/kg/day (administered as four doses per day) via subcutaneous injection to pregnant mice from gestation day (GD) 6 through 15 resulted in durlobactam-related, increased incidence of skeletal variations (unossified calcaneum) of the hindlimb and asymmetrical ossification of the sternebrae and supernumerary ribs at 800 and/or 1600 mg/kg, equivalent to 2- and 4-times the maximum recommended human dose based on area under the curve (AUC) comparisons. No adverse effects on mean body weight, reproductive performance, or cesarean section parameters were observed in any pregnant mice. IV infusion (2 hours/day) of durlobactam to pregnant female Sprague Dawley rats from GD 6 to weaning on Lactation Day 20 at a dose level of 300 or 1000 mg/kg/day was well tolerated with no adverse maternal effects in either group. Similarly, there was no adverse effect of maternal treatment in either group on embryo-fetal, perinatal or postnatal development up to 1000 mg/kg/day (approximately 4 times the MRHD based on AUC).
Lactation
Risk Summary
There are no data on the presence of durlobactam in human or animal milk. Sulbactam is present in human milk in low concentrations. Published data report sulbactam in breastmilk at an estimated maximum daily infant dose of 560 mcg/kg/day (1% to 2% of adult weight-adjusted dose), assuming mean milk consumption of 200 mL/kg/day. There are no data on the effects of XACDURO, sulbactam, or durlobactam on the breastfed infant or on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for XACDURO and any potential adverse effects on the breastfed child from XACDURO or from the underlying maternal condition.
Pediatric Use
The safety and effectiveness of XACDURO in pediatric patients younger than 18 years of age have not been established.
Geriatric Use
Of the 91 patients treated with XACDURO in Trial 1, 49 (54%) were 65 years of age and older, including 17 (19%) patients 76 years of age and older. Clinical studies of XACDURO did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients.
XACDURO is known to be substantially excreted by the kidney and elderly patients are more likely to have decreased renal function. Adjust dosing regimen for elderly patients based on renal function [see Dosage and Administration (2.2) , Use in Specific Populations (8.6) and Clinical Pharmacology (12.3) ] .
Renal Impairment
Patients with CLcr 45 to 129 mL/min
No dosage adjustment of XACDURO is recommended in patients with CLcr 45 to 129 mL/min.
Patients with CLcr Less Than 45 mL/min Including Patients Receiving Intermittent HD
Adjustments to the XACDURO dosing regimen are required in patients with CLcr less than 45 mL/min. In patients requiring HD, complete HD at the latest possible time before the start of XACDURO dosing [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3) ] . Monitor renal function regularly and adjust the dosage of XACDURO accordingly as renal function may change during the course of therapy.
Patients Receiving Continuous Renal Replacement Therapy (CRRT)
Limited information is available to provide a dosage recommendation in this setting and therapy should be guided by the patient's clinical status. While on CRRT, a patient's residual renal function may change, which may warrant a change in XACDURO dosage. Monitor renal function regularly and adjust the dosage of XACDURO accordingly as renal function may change during the course of therapy.
Patients with CLcr 130 mL/min or Greater
CLcr 130 mL/min or greater may be seen in seriously ill patients who are receiving intravenous fluid resuscitation. Dosage adjustment of XACDURO is required in patients with CLcr 130 mL/min or greater [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3) ] . Monitor renal function regularly and adjust the dosage of XACDURO accordingly as renal function may change during the course of therapy.
Hepatic Impairment
The effects of hepatic impairment on the pharmacokinetics of sulbactam and durlobactam have not been evaluated. Hepatic impairment is not expected to alter the elimination of XACDURO as neither sulbactam nor durlobactam undergo substantial hepatic metabolism/excretion. Dosage adjustments are not necessary in patients with impaired hepatic function.
CONTRAINDICATIONS
XACDURO is contraindicated in patients with a history of known severe hypersensitivity to the components of XACDURO (sulbactam and durlobactam), or other beta-lactam antibacterial drugs [see Warnings and Precautions (5.1) ] .
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions : Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported with beta-lactam antibacterial drugs. Hypersensitivity was observed in patients treated with XACDURO. If an allergic reaction occurs, discontinue XACDURO. (5.1 )
- Clostridioides difficile -Associated Diarrhea (CDAD): CDAD has been reported with nearly all systemic antibacterial agents, including XACDURO. Evaluate if diarrhea occurs. (5.2 )
Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions and serious skin reactions have been reported in patients receiving beta-lactam antibacterial drugs. These reactions are more likely to occur in individuals with a history of beta-lactam hypersensitivity and/or a history of sensitivity to multiple allergens. Hypersensitivity was observed in patients treated with XACDURO in clinical trials [see Adverse Reactions (6.1) ] . Before initiating therapy with XACDURO, careful inquiry should be made concerning previous hypersensitivity reactions to carbapenems, penicillins, cephalosporins, other beta lactams, and other allergens. Discontinue XACDURO if an allergic reaction occurs.
Clostridioides difficile -Associated Diarrhea (CDAD)
Clostridioides difficile -associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including XACDURO, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile .
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, the risk/benefit of continuing treatment with XACDURO should be assessed. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile , and surgical evaluation should be instituted as clinically indicated.
Development of Drug-Resistant Bacteria
Prescribing XACDURO in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
ADVERSE REACTIONS
The following serious adverse reactions are described in greater detail in the Warnings and Precautions section:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1) ]
- Clostridioides difficile -Associated Diarrhea (CDAD) [see Warnings and Precautions (5.2) ]
Clinical Trials Experience
Clinical trials are conducted under widely varying conditions, therefore adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of durlobactam with or without sulbactam was evaluated in 380 adult subjects across six phase 1 trials, one phase 2 trial in patients with complicated urinary tract infections (cUTIs) including acute pyelonephritis, and one phase 3 trial (also referred to as Trial 1) in adult patients with infections caused by Acinetobacter baumannii-calcoaceticus complex including hospital-acquired bacterial pneumonia (HABP), ventilator-associated bacterial pneumonia (VABP), and ventilated pneumonia (VP) [see Clinical Studies (14) ].
In the randomized, active-controlled portion of the phase 3 trial, 91 patients received XACDURO (1 g sulbactam and 1 g durlobactam, or renally adjusted dose) intravenously over 3 hours every 6 hours and 86 patients were treated with colistin 2.5 mg/kg (or renally adjusted dose) intravenously over 30 minutes every 12 hours after an initial loading dose of colistin 2.5 to 5 mg/kg. Both treatment arms also received 1 g imipenem/1 g cilastatin (or renally adjusted dose) intravenously every 6 hours as background therapy for potential HABP/VABP pathogens other than Acinetobacter baumannii-calcoaceticus complex. The mean duration of XACDURO therapy was 9 days versus 8 days for colistin.
Serious Adverse Reactions and Discontinuation of Treatment
Thirty-six patients (40%) in the XACDURO treatment group and 42 patients (49%) in the colistin treatment group experienced serious adverse reactions. Discontinuation of treatment due to any adverse reaction occurred in 10/91 (11%) patients treated with XACDURO and in 14/86 (16%) patients treated with colistin. One patient treated with XACDURO developed anaphylactic shock which led to discontinuation of treatment.
Common Adverse Reactions
Adverse reactions were reported in 88% (80/91) of patients in the XACDURO treatment group and 94% (81/86) of patients in the colistin treatment group. The most common adverse reactions reported in >10% of patients treated with XACDURO were liver test abnormalities, diarrhea, anemia, and hypokalemia. Table 2 lists selected adverse reactions occurring at a frequency of >5% in Trial 1.
| Adverse Reaction | XACDURO (N=91) n (%) | Colistin (N=86) n (%) |
|---|---|---|
| Any Adverse Reaction | 80 (88) | 81 (94) |
| Liver test abnormalities Liver test abnormalities includes the following adverse reactions: liver function test abnormal, hepatic function abnormal, increased transaminases, ALT increased, and AST increased; Acute kidney injury includes the following adverse reactions: renal impairment, blood Cr increased, toxic nephropathy, renal failure and acute kidney injury. | 17 (19) | 18 (21) |
| Diarrhea | 15 (17) | 9 (11) |
| Anemia | 12 (13) | 12 (14) |
| Hypokalemia | 11 (12) | 9 (11) |
| Arrhythmia | 8 (9) | 8 (9) |
| Acute kidney injury | 5 (6) | 31 (36) |
| Thrombocytopenia | 5 (6) | 3 (4) |
| Constipation | 5 (6) | 5 (6) |
DRUG INTERACTIONS
Organic Anion Transporter 1 (OAT1) Inhibitors : Concomitant administration with OAT1 inhibitors may increase plasma concentrations of XACDURO. Concomitant administration is not recommended. (7.1 )
Organic Anion Transporter 1 (OAT1) Inhibitors
Concomitant administration with OAT1 inhibitors may increase plasma concentrations of sulbactam. Concomitant administration of OAT1 inhibitors (e.g., probenecid) with XACDURO is not recommended. [see Clinical Pharmacology (12.3) ] .
DESCRIPTION
XACDURO (sulbactam for injection and durlobactam for injection) is an antibacterial co-packaged product containing sulbactam sodium, a penicillin derivative beta-lactam antibacterial and beta-lactamase inhibitor, and durlobactam sodium, a diazabicyclooctane beta-lactamase inhibitor, for intravenous administration.
Sulbactam Sodium
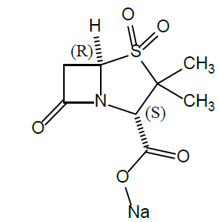
Sulbactam sodium is a beta-lactam antibacterial and a beta-lactamase inhibitor. Chemically, sulbactam sodium is sodium penicillinate sulfone; sodium (2S, 5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylate 4,4-dioxide. Sulbactam is a white to off-white crystalline powder that is freely soluble in water and its empirical formula is C 8 H 10 NNaO 5 S with a molecular weight of 255.22.
Figure 1: Chemical structure of sulbactam sodium
Durlobactam Sodium
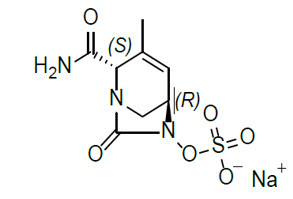
Durlobactam is a beta-lactamase inhibitor antibacterial drug. Chemically, durlobactam sodium is sodium (2 S ,5 R )-2-carbamoyl-3-methyl-7-oxo-1,6-diazabicyclo[3.2.1]oct-3-en-6-yl sulfate. Durlobactam sodium is the pure (2S,5R)-enantiomer of the trans diastereoisomer. It is a white to yellow amorphous powder that is freely soluble in water. The empirical formula is C 8 H 10 N 3 NaO 6 S and the molecular weight (sodium salt) is 299.23.
Figure 2: Chemical structure of durlobactam sodium
XACDURO is supplied as a co-packaged kit containing three single-dose vials each containing sterile powder for reconstitution: one clear single-dose vial contains 1 g sulbactam (equivalent to 1.1 g sulbactam sodium) and two amber single-dose vials each contain 0.5 g durlobactam (equivalent to 0.54 g durlobactam sodium) together with sodium hydroxide and hydrochloric acid used for pH adjustment.
CLINICAL PHARMACOLOGY
Mechanism of Action
XACDURO is an antibacterial drug [see Microbiology (12.4) ].
Pharmacodynamics
For sulbactam, the percent time of dosing interval that unbound plasma concentrations of sulbactam exceed the minimum inhibitory concentration (MIC) of A. baumannii has been shown to be the best predictor of efficacy in animal and in vitro models of infection. For durlobactam, the ratio of the 24-hour unbound plasma durlobactam AUC to the sulbactam-durlobactam MIC ( f AUC 0–24 /MIC) best predicts the activity in in vivo and in vitro models of infection.
Cardiac Electrophysiology
At a dose 4 times the maximum recommended single dose, durlobactam does not prolong the QTc interval to any clinically relevant extent.
Pharmacokinetics
Sulbactam and durlobactam pharmacokinetics are similar following single- and multiple-dose administrations. The C max and AUC of sulbactam increase in proportion to dose (0.5 times the recommended single dose to 1 g single dose). Durlobactam demonstrated dose-proportional pharmacokinetics across the dose range studied (0.25 times the recommended single dose to 2 times the recommended single dose infused over 3 hours every 6 hours).
The pharmacokinetic properties of the components of XACDURO and the pharmacokinetic parameters of sulbactam and durlobactam are summarized in Table 3.
| Pharmacokinetic Parameters | Sulbactam | Durlobactam |
|---|---|---|
| AUC 0-6 = area under the plasma concentration time curve from time of dosing to 6 hours post dose; AUC 0-24 = area under the plasma concentration time curve from time of dosing to 24 hours; CL = clearance; C max = maximum concentration; ELF = epithelial lining fluid; SD = standard deviation, T 1/2 = terminal half-life; V ss = volume of distribution at steady state | ||
| C max (µg/mL) Pharmacokinetic parameters are presented at steady state (Day 3) in patients with normal renal function defined as creatinine clearance of greater than or equal to 90 mL/min and less than 130 mL/min at a dosage of 1 g sulbactam and 1 g durlobactam every 6 hours. | 32.4 ± 24.7 | 29.2 ± 13.2 |
| AUC 0-24 (h∙µg/mL) | 515 ± 458 | 471 ± 240 |
| Distribution | ||
| % Bound to human plasma protein | 38% | 10% |
| AUC 0-6 ELF/plasma ratio | 0.5 | 0.37 |
| V ss (L) | 25.4 ± 11.3 | 30.3 ± 12.9 |
| Metabolism | Minimally metabolized | |
| Elimination | ||
| CL (L/h) | 11.6 ± 5.64 | 9.96 ± 3.11 |
| T 1/2 (h) | 2.15 ± 1.16 | 2.52 ± 0.77 |
| Excretion | ||
| Major route of elimination | Renal | |
| % excreted unchanged in urine | 75% to 85% | 78% |
Specific Populations
No clinically significant differences in the pharmacokinetics of sulbactam or durlobactam were observed based on age (18-91 years), gender, weight (35-150 kg), and race (White, Black, Asian, American Indian/Alaska Native, Other).
Patients with Renal Impairment
In a single-dose trial evaluating the effect of renal impairment on the pharmacokinetics of sulbactam and durlobactam, dose normalized systemic exposures of sulbactam and durlobactam were higher at all levels of renal impairment compared to healthy subjects with CLcr greater than or equal to 90 mL/min (Table 4). In subjects with end stage renal disease (ESRD) on hemodialysis, the fraction of the dose removed by hemodialysis was 0.41 and 0.33 for sulbactam and durlobactam, respectively.
| Estimated eGFR (mL/min/1.73 m 2 ) | Sulbactam | Durlobactam |
|---|---|---|
| ≥60 to <90 | 1.4 | 1.4 |
| ≥30 to <60 | 2.0 | 1.9 |
| <30 | 4.3 | 3.7 |
To maintain systemic exposures similar to patients with normal renal function, dosage adjustment is recommended for patients with renal impairment. Patients on hemodialysis should receive XACDURO after hemodialysis session [see Dosage and Administration (2.2) , Specific Populations (8.6) ] .
Patients with CLcr 130 mL/min or Greater
Increased sulbactam and durlobactam clearance have been observed in patients with CLcr of 130 mL/min or greater. A XACDURO (1.0 g sulbactam and 1.0 g durlobactam) dose every 4 hours infused over 3 hours provided sulbactam and durlobactam exposures comparable to those in patients with CLcr 90 to 129 mL/min [see Dosage and Administration (2.2) , Use in Specific Populations (8.6) ] .
Patients with Hepatic Impairment
Sulbactam and durlobactam are primarily cleared renally; therefore, hepatic impairment is not likely to have any effect on XACDURO exposures [see Use in Specific Populations (8.7) ] .
Drug Interaction Studies
Clinical Studies
No drug-drug interactions were observed among durlobactam, sulbactam, imipenem, and cilastatin in a clinical study in healthy subjects.
In Vitro Studies Where Drug Interaction Potential Was Not Further Evaluated Clinically Cytochrome P450 (CYP) Enzymes: At therapeutic plasma concentrations, durlobactam does not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4. Durlobactam showed no potential for in vitro induction of CYP1A2, CYP2B6, or CYP3A4.
No in vitro studies with CYP450 and sulbactam were conducted.
Membrane Transporter Systems:
In vitro data showed that sulbactam did not inhibit P-gp, BCRP, OATP1B1, OATP1B3, OCT1, OCT2, BSEP, OAT1, OAT3, MATE1, or MATE2-K at therapeutic plasma concentrations. In vitro data also indicated that durlobactam did not inhibit P-gp, BCRP, OATP1B1, OAT1, OAT3, or OCT2 at therapeutic plasma concentrations. Sulbactam and durlobactam are both substrates of OAT1. However, only sulbactam is predicted to have active secretion as a significant portion of total clearance; therefore, inhibition of OAT1 may increase sulbactam plasma concentrations. No clinical studies have been conducted with XACDURO and OAT1 inhibitors [see Drug Interactions (7.1) ] .
Microbiology
Mechanism of Action
XACDURO is a co-packaged product containing sulbactam and durlobactam. Sulbactam is a beta-lactam antibacterial and Ambler Class A serine beta-lactamase inhibitor that has bactericidal activity due to its inhibition of Acinetobacter baumannii-calcoaceticus complex (ABC) penicillin-binding proteins PBP1 and PBP3, which are essential enzymes required for bacterial cell wall synthesis.
Durlobactam is a diazabicyclooctane non-beta-lactam, beta-lactamase inhibitor, that protects sulbactam from degradation by certain serine-beta-lactamases. Durlobactam alone does not have antibacterial activity against ABC isolates.
XACDURO demonstrated in vitro activity against ABC isolates expressing serine beta-lactamases included in Ambler Class A (CTX-M-, TEM-, PER- and SHV-type extended spectrum beta-lactamases [ESBLs], KPC carbapenemase) Class C (ADC-type) and broad spectrum activity against Class D (OXA-type) enzymes.
Resistance
Mechanisms of beta-lactam resistance in ABC isolates may include either the production of beta-lactamases, modification of PBPs or target alteration, up-regulation of efflux pumps or loss of outer membrane porin.
XACDURO is not active against ABC isolates that produce Ambler Class B metallo-beta-lactamases or have modification of active target site of sulbactam (i.e., PBPs). Isolates may also produce in combination multiple beta-lactamases, express varying levels of beta-lactamases, have amino acid PBPs sequence variations or other resistance mechanisms that may contribute to resistance.
Interaction with Other Antimicrobials
In vitro studies showed no antagonism between XACDURO and other antibacterial drugs including ceftazidime-avibactam, imipenem, meropenem, amikacin, colistin, cefepime, ciprofloxacin, minocycline, rifampicin, rifaximin, daptomycin, dalbavancin, oritavancin, tedizolid, fidaxomicin, vancomycin, linezolid, metronidazole, and fluconazole.
Animal Models of Infection
Durlobactam restored activity of sulbactam in animal models of infection (including neutropenic mouse thigh and lung infections) against XACDURO-susceptible A. baumannii strains.
Antimicrobial Activity
XACDURO has been shown to be active against most isolates of the following microorganisms both in vitro and in clinical infections [see Indications and Usage (1.1) ].
- Acinetobacter baumannii-calcoaceticus complex
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for XACDURO, please see: https://www.fda.gov/STIC.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies with XACDURO have not been conducted.
Mutagenesis
Durlobactam was negative in genetic toxicology studies including the Salmonella typhimurium bacterial reverse mutation assay, in vivo and in vitro micronucleus assays, and the in vivo Pig-A assay in rats.
Genetic toxicology studies were not conducted with sulbactam or the combination of sulbactam-durlobactam.
Impairment of Fertility
No adverse effects on fertility, reproductive performance, fetal viability, growth, or postnatal development were observed in male and female rats, and female mice for durlobactam at intravenous doses up to 1000 mg/kg/day (approximately 4 times the MRHD based on AUC comparisons) and subcutaneous doses up to 1600 mg/kg/day, (equivalent to 4 times the MRHD based on AUC comparisons) respectively.
CLINICAL STUDIES
Treatment of Hospital-acquired and Ventilator-associated Bacterial Pneumonia Caused by Acinetobacter baumannii-calcoaceticus Complex Organisms
A total of 177 hospitalized adults with documented Acinetobacter baumannii-calcoaceticus complex infections were randomized and treated in a multicenter, active-controlled, investigator-unblinded, independent assessor-blinded, non-inferiority, phase 3 trial (Trial 1, NCT03894046). Patients were treated with either XACDURO (1 g sulbactam and 1 g durlobactam, or renally adjusted dose) intravenously over 3 hours every 6 hours (n = 91) or colistin 2.5 mg/kg (or renally adjusted dose) intravenously over 30 minutes every 12 hours after an initial loading dose of colistin 2.5 to 5 mg/kg (n = 86). Both treatment arms also received 1 g imipenem/1 g cilastatin (or renally adjusted dose) intravenously every 6 hours as background therapy for potential HABP/VABP pathogens other than Acinetobacter baumannii-calcoaceticus complex. Patients received up to 14 days of therapy.
The primary efficacy endpoint for the study was 28-day all-cause mortality in the patients who received any amount of study medication with a confirmed baseline infection with carbapenem-resistant Acinetobacter baumannii-calcoaceticus complex (CRABC microbiologically modified intent to treat (m-MITT) population). Among 128 patients in the CRABC m-MITT population, 125 patients who did not withdraw consent prior to assessment of survival status at Day 28 were assessed: 63 patients in the XACDURO group and 62 patients in the colistin group.
The demographic and baseline characteristics were comparable between treatment groups among 125 patients in the analysis: 74% male, 50% White, and 44% Asian; the mean age was 63 (±17) years. The majority of patients had pneumonia as the baseline infection (53% VABP and 43% HABP); 2% had bacteremia. The mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score at baseline was 17, and 26% of patients had an APACHE II score ≥ 20. At randomization, 64% of patients had been in the ICU ≥5 days, 26% of patients had been in the ICU for >14 days, and 75% were mechanically ventilated. Approximately 39% of patients had CLcr less than 90 mL/min at baseline. The mean duration of treatment was 9 days for the XACDURO group and 8 days for the colistin group.
Table 5 shows results for the primary endpoint of Day 28 all-cause mortality in the CRABC m-MITT population. XACDURO was non-inferior to colistin with regard to Day 28 all-cause mortality.
| XACDURO n/N Percentage was calculated using N, the number of patients in the specified population, as the denominator. (%) | Colistin n/N(%) | Treatment Difference (95% CI) The 95% CI (2-sided) was computed using a continuity-corrected z statistic. | |
|---|---|---|---|
| CI = Confidence Interval | |||
| Day 28 All-Cause Mortality | 12/63 (19.0%) | 20/62 (32.3%) | -13.2% (-30.0, 3.5) |
Clinical cure rates were also evaluated. Clinical cure was defined as complete resolution or significant improvement of signs and symptoms that were present at baseline and no new symptoms, such that no additional gram-negative antimicrobial therapy was warranted. Clinical cure rates in the CRABC m-MITT population at the Test of Cure (TOC) Visit that was 7 days (±2 days) after the end of treatment were 39/63 (61.9%) for XACDURO versus 25/62 (40.3%) for colistin.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
XACDURO is a co-packaged product containing sulbactam for injection and durlobactam for injection. XACDURO is supplied as a kit (NDC 68547-111-10) containing the following single-dose vials each containing sterile powder for reconstitution:
- 1 g sulbactam in one clear glass single-dose vial
- 1 g durlobactam in two amber glass single-dose vials (0.5 g durlobactam in each single-dose vial)
Stoppers are not made with natural rubber latex.
Storage and Handling
XACDURO vials should be stored refrigerated at 2°C to 8°C (36°F to 46°F); brief exposure to 8°C to 15°C (46°F to 59°F) permitted [see USP Controlled Cold Temperature]. Do not freeze. Store prepared XACDURO solution in the refrigerator [see Dosage and Administration (2.6) ].
INSTRUCTIONS FOR USE
| XACDURO ® (sulbactam for injection; durlobactam for injection), co-packaged for intravenous use 1 g/1 g per dose Single-dose kit 3 vials |  | Durlobactam 0.5 g/vial |
 | Durlobactam 0.5 g/vial | |
 | Sulbactam 1 g/vial | |
| This Instructions for Use provides information for preparing all 3 vials as a single dose . Read this Instructions for Use before using this vial kit and prior to each use. | ||
Important Information Before Injecting
Important Information
|
For product complaints or questions, please call 1-800-651-3861.
| Supplies | |||
| Included with kit: | Non-kit components: | ||
 (2) Durlobactam 0.5 g/vials (2) Durlobactam 0.5 g/vials |  | 100 mL of 0.9% sodium chloride infusion bag | |
 |  |  |  |
| (1) Sulbactam 1 g/vial | Alcohol wipes | Sterile water for injection | Syringe |
1 Prepare Supplies
| a.  | c.  |
d.  | e.  |
2 Clean Supplies
| a.  | b.  | c.  |
| Failure to clean could result in loss of sterility. | |||
| 3 |  | Reconstitute and Inject Both Durlobactam Vials (Vials 1 of 3 and 2 of 3) | ||||||
| ||||||||
| a. 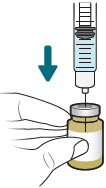 | b. 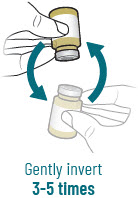 | c. 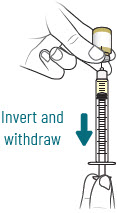 | d. 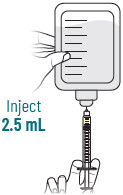 | e.  | |||
| After reconstituting, ensure there are no visisble particles in the solution. | ||||||||
| ||||||||
| 4 |  | Reconstitute and Inject Sulbactam Vial (Vial 3 of 3) | |||||
| a. 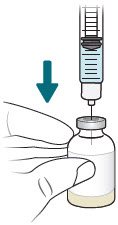 | b. 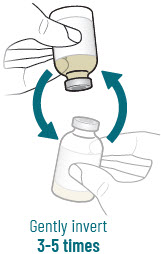 | c. 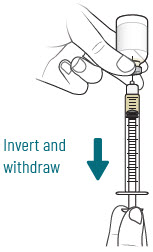 | d. 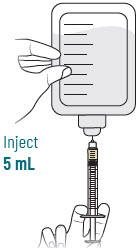 | |||
| After reconstituting, ensure there are no visible particles in the solution. | |||||||
| |||||||
5 Prepare Infusion Bag
| 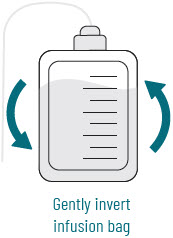 | 6 Store Infusion Bag
| a. 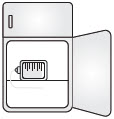 | 7 Dispose of Materials
|  |
| Note: Before infusing, allow 15-30 minutes for infusion bag to reach room temperature after removing from refrigerator. | b.  |
XACDURO ® is a registered trademark of Entasis Therapeutics Limited.
© 2023 Entasis Therapeutics. All rights reserved.
INS16343 01 Approved 2023 MAY
Mechanism of Action
XACDURO is an antibacterial drug [see Microbiology (12.4) ].