Xofluza prior authorization resources
Most recent state uniform prior authorization forms
Xofluza patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
Treatment and Post-Exposure Prophylaxis of Influenza
- Take XOFLUZA as a single dose as soon as possible and within 48 hours of influenza symptom onset for treatment of acute uncomplicated influenza or following contact with an individual who has influenza. XOFLUZA may be taken with or without food. (2.1 , 2.2 )
| Patient Body Weight | Recommended Single Oral Dose in Patients 5 Years of Age and Older (Tablets) |
|---|---|
| 20 kg to less than 80 kg | One 40 mg tablet (blister card contains one 40 mg tablet) |
| At least 80 kg | One 80 mg tablet (blister card contains one 80 mg tablet) |
| Patient Body Weight | Recommended Single Oral Dose in Patients 5 Years of Age and Older For Oral Suspension (Packets) |
|---|---|
| 15 kg to less than 20 kg | One 30 mg packet |
| 20 kg to less than 80 kg | One 40 mg packet |
| At least 80 kg | 80 mg (two 40 mg packets) |
| Patient Body Weight | Recommended Single Oral Dose in Patients 5 Years of Age and Older For Oral Suspension (Bottles) |
|---|---|
| Less than 20 kg | 2 mg/kg |
| 20 kg to less than 80 kg | 40 mg (20 mL) |
| At least 80 kg | 80 mg (40 mL) |
Refer to the Full Prescribing Information for additional information on the recommended dosage and preparation of XOFLUZA for oral suspension (bottles) or for oral suspension (packets) for both for oral or enteral use in patients 5 years of age and older. (2.2 , 2.3 )
Dosage and Administration Overview
XOFLUZA is available in two dosage forms:
- XOFLUZA tablets (40 mg and 80 mg).
- XOFLUZA for oral suspension is available in two different presentations: packets (30 mg and 40 mg) and bottles (2 mg/mL). If the patient weighs less than 15 kg, XOFLUZA for oral suspension in bottle is the recommended presentation. Both presentations of the for oral suspension are intended for patients who are unable to or have difficulty swallowing tablets, or those who require enteral administration [see Dosage and Administration (2.2 , 2.3 , 2.4) ] .
Take XOFLUZA as soon as possible after influenza symptom onset or exposure to influenza [see Dosage and Administration (2.2) ] .
XOFLUZA may be taken with or without food. However, concomitant use of XOFLUZA with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives, antacids, or oral supplements (e.g., calcium, iron, magnesium, selenium, or zinc) should be avoided [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) ].
Recommended Dosage
Treatment of Acute Uncomplicated Influenza or Post-Exposure Prophylaxis in Adults, and Pediatric Patients (5 Years of Age and Older)
Take XOFLUZA as a single dose as soon as possible and within 48 hours of influenza symptom onset for treatment of acute uncomplicated influenza or following contact with an individual who has influenza. The recommended dosage of XOFLUZA in patients 5 years of age or older is a single weight-based dose displayed in Tables 1 , 2 and 3 .
| Patient Body Weight | Recommended Single Oral Dose (Tablets) Recommended XOFLUZA dosage is based on the patient's weight. |
|---|---|
| 20 kg to less than 80 kg | One 40 mg tablet (blister card contains one 40 mg tablet) |
| At least 80 kg | One 80 mg tablet (blister card contains one 80 mg tablet) |
| Patient Body Weight | Recommended Single Oral Dose (Packets) Recommended XOFLUZA dosage is based on the patient's weight. |
|---|---|
| 15 kg Patients who weigh less than 15 kg should receive XOFLUZA for oral suspension (bottles). to less than 20 kg | One 30 mg packet |
| 20 kg to less than 80 kg | One 40 mg packet |
| At least 80 kg | 80 mg (two 40 mg packets) |
| Patient Body Weight | Recommended Single Oral Dose Recommended XOFLUZA dosage is based on the patient's weight. , Use a measuring device (oral syringe) to measure the prescribed dose for use. (For Oral Suspension in Bottles) |
|---|---|
| Less than 20 kg | 2 mg/kg |
| 20 kg to less than 80 kg | 40 mg (20 mL) |
| At least 80 kg | 80 mg (40 mL Dosage requires two bottles of XOFLUZA for oral suspension. ) |
Preparation of XOFLUZA for Oral Suspension (Packets) by Patient or Caregiver
See the XOFLUZA for oral suspension (packets) Instructions for Use for details on the preparation and administration (oral or via enteral feeding tube).
- For the 30 mg or 40 mg dose, mix in a small container with 1 tablespoon (about 15-20 mL) of room temperature drinking water. Once the supplied XOFLUZA for oral suspension (packets) have fully dispersed in the drinking water, take the entire mixture immediately.
- For the 80 mg dose, use two 40 mg packets. Prepare each packet as described above for the 40 mg dose and take the packets separately.
- For enteral administration (i.e., feeding tube), draw up the entire contents with an enteral syringe and administer through a tube that is 4 French or larger. Flush with 1 mL of water before and after enteral administration.
Preparation of XOFLUZA for Oral Suspension (Bottles) by Healthcare Provider
Prior to dispensing to the patient, reconstitute XOFLUZA for oral suspension (bottles) with 20 mL of drinking water or sterile water. After reconstitution, each bottle of XOFLUZA for oral suspension (bottles) contains 40 mg of baloxavir marboxil per 20 mL of volume for a final concentration of 2 mg/mL. The for oral suspension (bottles) can be used for oral or enteral use. Only take the contents of the full bottle(s) of XOFLUZA for oral suspension (bottles) with the use of a measuring device (oral syringe). Ensure the caregiver or patient uses an oral syringe to measure the prescribed dose of XOFLUZA for oral suspension (bottles). Patients may need to draw up XOFLUZA for oral suspension (bottles) multiple times using the oral syringe to receive the full dose.
Reconstituting XOFLUZA for Oral Suspension (Bottles)
Prepare the for oral suspension (bottles) at the time of dispensing. Administration must occur within 10 hours after reconstitution because this product does not contain a preservative.
- Gently tap the bottom of the bottle to loosen the granules.
- Reconstitute XOFLUZA for oral suspension (bottles) with 20 mL of drinking water or sterile water.
- Gently swirl the suspension to ensure that the granules are evenly suspended. Do not shake.
- Write the expiration time and date on the bottle label in the space provided (10 hours from reconstitution time).
Important Information for the Healthcare Provider
- Provide caregiver or patient with a measuring device (oral syringe) to deliver the prescribed dose of the for suspension for oral use (bottles). For enteral administration (i.e., feeding tube), draw up for oral suspension (bottles) with an enteral syringe and administer through a tube that is 4 French or larger. Flush with 1 mL of water before and after enteral administration.
- Instruct the caregiver or patient that the total prescribed dose of XOFLUZA for oral suspension (bottles) may require:
- Less than one bottle (e.g., for pediatric patients 5 years of age and older who weigh less than 20 kg)
- One bottle (e.g., for adults and adolescents weighing 20 kg to less than 80 kg), or
- Two bottles (e.g., for adults and adolescents weighing at least 80 kg).

By using PrescriberAI, you agree to the AI Terms of Use.
Xofluza prescribing information
INDICATIONS AND USAGE
XOFLUZA is an influenza virus polymerase acidic (PA) endonuclease inhibitor indicated for:
- Treatment of acute uncomplicated influenza in patients 5 years of age and older who have been symptomatic for no more than 48 hours and who are otherwise healthy or at high risk of developing influenza-related complications . (1.1 )
- Post-exposure prophylaxis of influenza in patients 5 years of age and older following contact with an individual who has influenza. (1.2 )
Limitations of Use
Influenza viruses change over time, and factors such as the virus type or subtype, emergence of resistance, or changes in viral virulence could diminish the clinical benefit of antiviral drugs. Consider available information on drug susceptibility patterns for circulating influenza virus strains when deciding whether to use XOFLUZA. (1.3 )
Treatment of Influenza
XOFLUZA is indicated for treatment of acute uncomplicated influenza in patients 5 years of age and older who have been symptomatic for no more than 48 hours and who are otherwise healthy or at high risk of developing influenza-related complications 1 [see Clinical Studies (14.1 , 14.2 , and 14.3 ].
Post-Exposure Prophylaxis of Influenza
XOFLUZA is indicated for post-exposure prophylaxis of influenza in persons 5 years of age and older following contact with an individual who has influenza [see Clinical Studies (14.4) ].
Limitations of Use
Influenza viruses change over time, and factors such as the virus type or subtype, emergence of resistance, or changes in viral virulence could diminish the clinical benefit of antiviral drugs. Consider available information on drug susceptibility patterns for circulating influenza virus strains when deciding whether to use XOFLUZA [see Warnings and Precautions (5.2) , Microbiology (12.4) and Clinical Studies (14) ] .
DOSAGE AND ADMINISTRATION
Treatment and Post-Exposure Prophylaxis of Influenza
- Take XOFLUZA as a single dose as soon as possible and within 48 hours of influenza symptom onset for treatment of acute uncomplicated influenza or following contact with an individual who has influenza. XOFLUZA may be taken with or without food. (2.1 , 2.2 )
| Patient Body Weight | Recommended Single Oral Dose in Patients 5 Years of Age and Older (Tablets) |
|---|---|
| 20 kg to less than 80 kg | One 40 mg tablet (blister card contains one 40 mg tablet) |
| At least 80 kg | One 80 mg tablet (blister card contains one 80 mg tablet) |
| Patient Body Weight | Recommended Single Oral Dose in Patients 5 Years of Age and Older For Oral Suspension (Packets) |
|---|---|
| 15 kg to less than 20 kg | One 30 mg packet |
| 20 kg to less than 80 kg | One 40 mg packet |
| At least 80 kg | 80 mg (two 40 mg packets) |
| Patient Body Weight | Recommended Single Oral Dose in Patients 5 Years of Age and Older For Oral Suspension (Bottles) |
|---|---|
| Less than 20 kg | 2 mg/kg |
| 20 kg to less than 80 kg | 40 mg (20 mL) |
| At least 80 kg | 80 mg (40 mL) |
Refer to the Full Prescribing Information for additional information on the recommended dosage and preparation of XOFLUZA for oral suspension (bottles) or for oral suspension (packets) for both for oral or enteral use in patients 5 years of age and older. (2.2 , 2.3 )
Dosage and Administration Overview
XOFLUZA is available in two dosage forms:
- XOFLUZA tablets (40 mg and 80 mg).
- XOFLUZA for oral suspension is available in two different presentations: packets (30 mg and 40 mg) and bottles (2 mg/mL). If the patient weighs less than 15 kg, XOFLUZA for oral suspension in bottle is the recommended presentation. Both presentations of the for oral suspension are intended for patients who are unable to or have difficulty swallowing tablets, or those who require enteral administration [see Dosage and Administration (2.2 , 2.3 , 2.4) ] .
Take XOFLUZA as soon as possible after influenza symptom onset or exposure to influenza [see Dosage and Administration (2.2) ] .
XOFLUZA may be taken with or without food. However, concomitant use of XOFLUZA with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives, antacids, or oral supplements (e.g., calcium, iron, magnesium, selenium, or zinc) should be avoided [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) ].
Recommended Dosage
Treatment of Acute Uncomplicated Influenza or Post-Exposure Prophylaxis in Adults, and Pediatric Patients (5 Years of Age and Older)
Take XOFLUZA as a single dose as soon as possible and within 48 hours of influenza symptom onset for treatment of acute uncomplicated influenza or following contact with an individual who has influenza. The recommended dosage of XOFLUZA in patients 5 years of age or older is a single weight-based dose displayed in Tables 1 , 2 and 3 .
| Patient Body Weight | Recommended Single Oral Dose (Tablets) Recommended XOFLUZA dosage is based on the patient's weight. |
|---|---|
| 20 kg to less than 80 kg | One 40 mg tablet (blister card contains one 40 mg tablet) |
| At least 80 kg | One 80 mg tablet (blister card contains one 80 mg tablet) |
| Patient Body Weight | Recommended Single Oral Dose (Packets) Recommended XOFLUZA dosage is based on the patient's weight. |
|---|---|
| 15 kg Patients who weigh less than 15 kg should receive XOFLUZA for oral suspension (bottles). to less than 20 kg | One 30 mg packet |
| 20 kg to less than 80 kg | One 40 mg packet |
| At least 80 kg | 80 mg (two 40 mg packets) |
| Patient Body Weight | Recommended Single Oral Dose Recommended XOFLUZA dosage is based on the patient's weight. , Use a measuring device (oral syringe) to measure the prescribed dose for use. (For Oral Suspension in Bottles) |
|---|---|
| Less than 20 kg | 2 mg/kg |
| 20 kg to less than 80 kg | 40 mg (20 mL) |
| At least 80 kg | 80 mg (40 mL Dosage requires two bottles of XOFLUZA for oral suspension. ) |
Preparation of XOFLUZA for Oral Suspension (Packets) by Patient or Caregiver
See the XOFLUZA for oral suspension (packets) Instructions for Use for details on the preparation and administration (oral or via enteral feeding tube).
- For the 30 mg or 40 mg dose, mix in a small container with 1 tablespoon (about 15-20 mL) of room temperature drinking water. Once the supplied XOFLUZA for oral suspension (packets) have fully dispersed in the drinking water, take the entire mixture immediately.
- For the 80 mg dose, use two 40 mg packets. Prepare each packet as described above for the 40 mg dose and take the packets separately.
- For enteral administration (i.e., feeding tube), draw up the entire contents with an enteral syringe and administer through a tube that is 4 French or larger. Flush with 1 mL of water before and after enteral administration.
Preparation of XOFLUZA for Oral Suspension (Bottles) by Healthcare Provider
Prior to dispensing to the patient, reconstitute XOFLUZA for oral suspension (bottles) with 20 mL of drinking water or sterile water. After reconstitution, each bottle of XOFLUZA for oral suspension (bottles) contains 40 mg of baloxavir marboxil per 20 mL of volume for a final concentration of 2 mg/mL. The for oral suspension (bottles) can be used for oral or enteral use. Only take the contents of the full bottle(s) of XOFLUZA for oral suspension (bottles) with the use of a measuring device (oral syringe). Ensure the caregiver or patient uses an oral syringe to measure the prescribed dose of XOFLUZA for oral suspension (bottles). Patients may need to draw up XOFLUZA for oral suspension (bottles) multiple times using the oral syringe to receive the full dose.
Reconstituting XOFLUZA for Oral Suspension (Bottles)
Prepare the for oral suspension (bottles) at the time of dispensing. Administration must occur within 10 hours after reconstitution because this product does not contain a preservative.
- Gently tap the bottom of the bottle to loosen the granules.
- Reconstitute XOFLUZA for oral suspension (bottles) with 20 mL of drinking water or sterile water.
- Gently swirl the suspension to ensure that the granules are evenly suspended. Do not shake.
- Write the expiration time and date on the bottle label in the space provided (10 hours from reconstitution time).
Important Information for the Healthcare Provider
- Provide caregiver or patient with a measuring device (oral syringe) to deliver the prescribed dose of the for suspension for oral use (bottles). For enteral administration (i.e., feeding tube), draw up for oral suspension (bottles) with an enteral syringe and administer through a tube that is 4 French or larger. Flush with 1 mL of water before and after enteral administration.
- Instruct the caregiver or patient that the total prescribed dose of XOFLUZA for oral suspension (bottles) may require:
- Less than one bottle (e.g., for pediatric patients 5 years of age and older who weigh less than 20 kg)
- One bottle (e.g., for adults and adolescents weighing 20 kg to less than 80 kg), or
- Two bottles (e.g., for adults and adolescents weighing at least 80 kg).
DOSAGE FORMS AND STRENGTHS
XOFLUZA Tablets:
- 40 mg: white to light yellow, oblong-shaped, film-coated, debossed with "BXM40" on one side.
- 80 mg: white to light yellow, oblong shaped, film-coated, debossed with "BXM80" on one side.
XOFLUZA for Oral Suspension (Packets):
- 30 mg: white to light yellow granules with strawberry flavor (each packet contains 30 mg baloxavir marboxil).
- 40 mg: white to light yellow granules with strawberry flavor (each packet contains 40 mg baloxavir marboxil).
XOFLUZA for Oral Suspension (Bottles):
- 40 mg/20 mL (2 mg/mL) of baloxavir marboxil after reconstitution with 20 mL of drinking water or sterile water. The granules are white to light yellow. The reconstituted product is a greyish white, white to light yellow opaque suspension with strawberry flavor.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
There are no adequate and well-controlled studies with XOFLUZA in pregnant women to inform a drug-associated risk of adverse developmental outcomes. There are risks to the mother and fetus associated with influenza virus infection in pregnancy (see Clinical Considerations ). In animal reproduction studies, no adverse developmental effects were observed in rats or rabbits with oral administration of baloxavir marboxil at exposures approximately 5 (rats) and 7 (rabbits) times the systemic baloxavir exposure at the maximum recommended human dose (MRHD) (see Data ) .
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%–4% and 15%–20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnant women are at higher risk of severe complications from influenza, which may lead to adverse pregnancy and/or fetal outcomes, including maternal death, stillbirth, birth defects, preterm delivery, low birth weight, and small for gestational age.
Data
Animal Data
Baloxavir marboxil was administered orally to pregnant rats (20, 200, or 1,000 mg/kg/day from gestation day 6 to 17) and rabbits (30, 100, or 1,000 mg/kg/day from gestation day 7 to 19). No adverse embryo-fetal effects were observed in rats up to the highest dose of baloxavir marboxil (1,000 mg/kg/day), resulting in systemic baloxavir exposure (AUC) of approximately 5 times the exposure at the MRHD. In rabbits, fetal skeletal variations occurred at a maternally toxic dose (1,000 mg/kg/day) resulting in 2 abortions out of 19 pregnancies. No adverse maternal or embryo-fetal effects were observed in rabbits at the middle dose (100 mg/kg/day) resulting in systemic baloxavir exposure (AUC) approximately 7 times the exposure at the MRHD.
In the prenatal and postnatal development study in rats, baloxavir marboxil was administered orally at 20, 200, or 1,000 mg/kg/day from gestation day 6 to postpartum/lactation day 20. No significant effects were observed in the offspring at maternal systemic baloxavir exposure (AUC) approximately 5 times the exposure at the MRHD.
Lactation
Risk Summary
There are no data on the presence of baloxavir marboxil in human milk, the effects on the breastfed infant, or the effects on milk production. Baloxavir and its related metabolites were present in the milk of lactating rats (see Data ) . The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for XOFLUZA and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition.
Data
In a lactation study, baloxavir and its related metabolites were excreted in the milk of lactating rats administered baloxavir marboxil (1 mg/kg) on postpartum/lactation day 11, with peak milk concentration approximately 5 times that of maternal plasma concentrations occurring 2 hours post-dose. No effects of baloxavir marboxil on growth and postnatal development were observed in nursing pups at the highest oral dose tested in rats. Maternal systemic exposure was approximately 5 times the baloxavir exposure in humans at the MRHD.
Pediatric Use
Treatment of Acute Uncomplicated Influenza in Adolescent Subjects (≥ 12 Years of Age)
The safety and effectiveness of XOFLUZA for the treatment of acute uncomplicated influenza in adolescent subjects 12 years of age and older weighing at least 40 kg is supported by one randomized, double-blind, controlled trial in otherwise healthy subjects Trial T0831 and one trial in subjects at high risk of developing influenza-related complications Trial T0832 [see Clinical Studies (14.1) ]. A total of 117 otherwise healthy adolescents 12 to17 years of age were randomized and received either XOFLUZA (N=76) or placebo (N=41) in Trial T0831; 38 adolescents 12 to 17 years of age at high risk for influenza complications were randomized and received either XOFLUZA (N=21) or placebo (N=17) in Trial T0832. The median time to alleviation of symptoms in influenza-infected adolescent subjects aged 12 to 17 years in Trial T0831 was comparable to that observed in adults. In Trial T0832, the median time to improvement of symptoms in the limited number of influenza-infected adolescent subjects aged 12 to 17 years was similar in the XOFLUZA and placebo arms [see Clinical Studies (14.1) ]. Adverse events reported in adolescents in both trials were similar to those reported in adults [see Adverse Reactions (6.1) ].
Treatment of Acute Uncomplicated Influenza in Pediatric Subjects (5 to < 12 Years of Age)
The safety and effectiveness of XOFLUZA in pediatric subjects 5 to less than 12 years of age is supported by one randomized, double-blind, controlled trial Trial CP40563 with a primary endpoint of safety. In this trial, 118 pediatric subjects were randomized and treated in a 2:1 ratio and received either XOFLUZA (N=79) or oseltamivir (N=39). Efficacy was extrapolated from adults and adolescents based on comparable PK exposures in adults, adolescents and pediatric subjects 5 to less than 12 years of age. The median time to alleviation of signs and symptoms in influenza-infected subjects was comparable in the XOFLUZA and oseltamivir arms. Adverse events reported with XOFLUZA in pediatric subjects were similar to those observed in adults and adolescents except for vomiting and diarrhea, which were both more commonly reported in pediatric subjects [see Adverse Reactions (6.1) , Clinical Pharmacology (12.3) , and Clinical Studies (14.1) ].
Post-Exposure Prophylaxis of Influenza in Pediatric and Adolescent Subjects (5 to < 18 Years of Age)
The safety and effectiveness of XOFLUZA for post-exposure prophylaxis in pediatric and adolescent subjects 5 to less than 18 years of age is supported by one randomized, double-blind, controlled trial conducted in Japan Trial T0834 [see Clinical Studies (14.3) ]. Subjects in this trial were randomized in a 1:1 ratio to receive XOFLUZA or placebo. A total of 69 subjects from 5 to <18 years of age in Trial T0834 received XOFLUZA. The incidence of RT-PCR-confirmed symptomatic influenza in pediatric subjects 5 to <18 years of age was similar to that observed in adult subjects [see Clinical Pharmacology (12.3) , and Clinical Studies (14.3) ]. Efficacy was extrapolated from adults based on comparable PK exposures in adults, adolescents and pediatric subjects 5 to <18 years of age.
Adverse events reported in pediatric and adolescent subjects were similar to those reported in adults in the same trial [see Adverse Reactions (6.1) ].
Pediatric Subjects (< 5 Years of Age)
The safety and effectiveness of XOFLUZA for treatment and post-exposure prophylaxis of influenza in pediatric subjects less than 5 years of age, including neonates, have not been established [see Warnings and Precautions (5.2) and Microbiology (12.4) ].
Geriatric Use
The safety and effectiveness of XOFLUZA in subjects 65 years of age and older has been established and is supported by one randomized, double-blind, controlled trial [see Clinical Studies (14.2) ] . In Trial T0832, of 730 XOFLUZA-treated subjects at high risk of influenza-related complications, 209 (29%) subjects were 65 years of age and older. The median time to improvement of influenza symptoms in subjects 65 years of age and older was 70 hours in subjects who received XOFLUZA (N=112) and 88 hours in those who received placebo (N=102). The safety profile observed for this population was similar to that reported in the overall trial population except for nausea, which was reported in 6% of elderly subjects compared to 1% of subjects from 18 to 64 years of age.
CONTRAINDICATIONS
XOFLUZA is contraindicated in patients with a history of hypersensitivity to baloxavir marboxil or any of its ingredients. Serious allergic reactions have included anaphylaxis, angioedema, urticaria, and erythema multiforme [see Warnings and Precautions (5.1) ].
WARNINGS AND PRECAUTIONS
- Hypersensitivity such as anaphylaxis, angioedema, urticaria, and erythema multiforme: Initiate appropriate treatment if an allergic-like reaction occurs or is suspected. (5.1 )
- Increased incidence of Treatment-Emergent Resistance in Patients Less Than 5 Years of Age: XOFLUZA is not indicated in patients less than 5 years of age due to increased incidence of treatment-emergent resistance in this age group . In clinical trials, incidence of virus with treatment-emergent substitutions associated with reduced susceptibility to baloxavir (resistance) was higher in pediatric subjects younger than 5 years of age than older subjects. (5.2 )
- Risk of bacterial infection: Serious bacterial infections may begin with influenza-like symptoms or may coexist with, or occur as, a complication of influenza. XOFLUZA has not been shown to prevent such complications. Prescribers should be alert to potential secondary bacterial infections and treat them as appropriate. (5.3 )
Hypersensitivity
Cases of anaphylaxis, urticaria, angioedema, and erythema multiforme have been reported in postmarketing experience with XOFLUZA. Appropriate treatment should be instituted if an allergic-like reaction occurs or is suspected. The use of XOFLUZA is contraindicated in patients with known hypersensitivity to XOFLUZA [see Contraindications (4) and Adverse Reactions (6.2) ].
Increased Incidence of Treatment-Emergent Resistance in Patients Less Than 5 Years of Age
XOFLUZA is not indicated in patients less than 5 years of age due to increased incidence of treatment-emergent resistance in this age group. In clinical trials, the incidence of virus with treatment-emergent substitutions associated with reduced susceptibility to baloxavir (resistance) was higher in pediatric subjects younger than 5 years of age (40%, 38/96) than in pediatric subjects ≥ 5 years to < 12 years of age (16%, 19/117) or subjects ≥ 12 years of age (7%, 60/842). The potential for transmission of resistant strains in the community has not been determined [see Indications and Usage (1) , Use in Specific Populations (8.4) , and Microbiology (12.4) ].
Risk of Bacterial Infections
There is no evidence of efficacy of XOFLUZA in any illness caused by pathogens other than influenza viruses. Serious bacterial infections may begin with influenza-like symptoms or may coexist with, or occur as, a complication of influenza. XOFLUZA has not been shown to prevent such complications. Prescribers should be alert to potential secondary bacterial infections and treat them as appropriate.
ADVERSE REACTIONS
Adverse events reported in at least 1% of adult and adolescent influenza subjects treated with XOFLUZA included diarrhea (3%), bronchitis (3%), nausea (2%), sinusitis (2%), and headache (1%). (6.1 )
Adverse events reported in at least 5% of pediatric subjects (5 to < 12 years) treated with XOFLUZA included vomiting (5%) and diarrhea (5%).
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The overall safety profile of XOFLUZA is based on data from 2,079 subjects, 5 years of age and older in 5 controlled clinical trials who received XOFLUZA. Of these subjects, 1,943 were adults and adolescents (≥ 12 years of age) and 136 were in the pediatric age group (5 to < 12 years of age) [see Clinical Studies (14) ] .
Treatment of Acute Uncomplicated Influenza
Adult and Adolescent Subjects (≥ 12 Years of Age):
The safety of XOFLUZA in adult and adolescent subjects is based on data from 3 placebo-controlled trials in which a total of 1,640 subjects received XOFLUZA: 1,334 (81%) subjects were 18 to 64 years of age, 209 (13%) subjects were adults 65 years of age or older, and 97 (6%) subjects were adolescents 12 to 17 years of age. These trials included otherwise healthy adults and adolescents (N=910) and subjects at high risk of developing complications associated with influenza (N=730). Of these, 1,440 subjects received XOFLUZA at the recommended dose [see Clinical Studies (14.1 , 14.2) ]. Trial T0821 was a phase 2 dose-finding placebo-controlled trial where otherwise healthy adult subjects 20 to 64 years of age received single oral dose of XOFLUZA or placebo. Trial T0831 was a placebo- and active-controlled trial in otherwise healthy adults and adolescents 12 to 64 years of age; subjects received weight-based XOFLUZA or placebo as a single oral dose on Day 1 or oseltamivir twice a day for 5 days. Trial T0832 was a randomized, double-blind, placebo- and active-controlled trial where adults and adolescents at high risk of influenza complications 12 years of age and older received either XOFLUZA, placebo or oseltamivir.
Table 4 displays the most common adverse events (regardless of causality assessment) reported in at least 1% of adult and adolescent subjects who received XOFLUZA at the recommended dose in Trials T0821, T0831, and T0832.
| Adverse Event | XOFLUZA (N=1,440) | Placebo (N=1,136) |
|---|---|---|
| Diarrhea | 3% | 4% |
| Bronchitis | 3% | 4% |
| Nausea | 2% | 3% |
| Sinusitis | 2% | 3% |
| Headache | 1% | 1% |
Pediatric Subjects (5 to < 12 Years of Age):
In an active-controlled, double-blind trial Trial CP40563 in pediatric subjects, a total of 79 subjects 5 to less than 12 years of age, received the recommended weight-based dosage of XOFLUZA, and 39 subjects received oseltamivir. Of the 118 subjects 5 to less than 12 years of age in Trial CP40563, 15 subjects in the XOFLUZA arm and 4 subjects in the oseltamivir arm were at high risk of developing influenza complications. The most frequently reported AEs (≥ 5%) in all subjects in the XOFLUZA treatment arm were vomiting (5%) and diarrhea (5%). Vomiting was reported in 18% of subjects in the oseltamivir arm [see Clinical Studies (14.3 ]. There are limited safety data in patients 5 to <12 years at high risk of developing influenza complications.
Post-Exposure Prophylaxis of Influenza
In a placebo-controlled clinical trial, Trial T0834, conducted in adults, and pediatric subjects ≥ 5 years of age, a total of 360 subjects received XOFLUZA, of which 291 (81%) were adults ≥ 18 years; 12 (3%) subjects were adolescents ≥ 12 to 17 years and 57 (16%) were pediatric subjects 5 to < 12 years of age. The safety profile was similar in pediatric patients aged 5 to < 12 years old as that reported in adults and adolescents 12 years of age and older [see Clinical Studies (14.4) ].
Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of XOFLUZA. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to XOFLUZA exposure.
Immune System Disorders: Anaphylactic reactions, anaphylactic shock, anaphylactoid reactions, hypersensitivity reactions, angioedema (swelling of face, eyelids, tongue and lips)
Skin and Subcutaneous Tissue Disorders: Rash, urticaria, erythema multiforme
Gastrointestinal Disorders: Vomiting, hematochezia, melena, colitis
Psychiatric Disorders: Delirium, abnormal behavior, hallucinations
DRUG INTERACTIONS
Effect of Other Drugs on XOFLUZA
Baloxavir may form a chelate with polyvalent cations such as calcium, aluminum, or magnesium. Coadministration with polyvalent cation-containing products may decrease plasma concentrations of baloxavir [see Clinical Pharmacology (12.3) ], which may reduce XOFLUZA efficacy. Avoid coadministration of XOFLUZA with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives, antacids, or oral supplements (e.g., calcium, iron, magnesium, selenium, or zinc).
Vaccines
The concurrent use of XOFLUZA with intranasal live attenuated influenza vaccine (LAIV) has not been evaluated. Concurrent administration of antiviral drugs may inhibit viral replication of LAIV and thereby decrease the effectiveness of LAIV vaccination. Interactions between inactivated influenza vaccines and XOFLUZA have not been evaluated.
DESCRIPTION
Baloxavir marboxil is an influenza virus PA endonuclease inhibitor.
The active component of XOFLUZA is baloxavir marboxil. The chemical name of baloxavir marboxil is ({(12aR)-12-[(11S)-7,8-Difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl]-6,8-dioxo-3,4,6,8,12,12a-hexahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl}oxy)methyl methyl carbonate. The empirical formula of baloxavir marboxil is C 27 H 23 F 2 N 3 O 7 S, and the chemical structure is shown below.
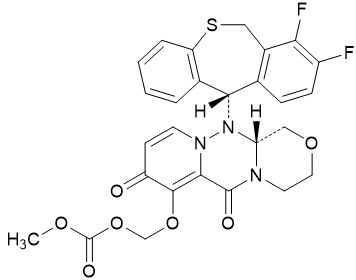
Baloxavir marboxil has a molecular mass of 571.55 grams per mole and a partition coefficient (log P) of 2.26. It is freely soluble in dimethylsulfoxide, soluble in acetonitrile, slightly soluble in methanol and ethanol, and practically insoluble in water.
XOFLUZA is supplied as tablets and for oral suspension (in packets and in bottles).
XOFLUZA tablets are white to light yellow, film-coated for oral administration . The 40 mg film-coated tablet contains 40 mg of baloxavir marboxil, and the 80 mg film-coated tablet contains 80 mg of baloxavir marboxil. The inactive ingredients of XOFLUZA tablets are: croscarmellose sodium, hypromellose, lactose monohydrate, microcrystalline cellulose, povidone K25, sodium stearyl fumarate, talc, and titanium dioxide.
XOFLUZA for oral suspension (packets) is supplied as white to light yellow granules in a packet. Each packet contains either 30 mg or 40 mg of baloxavir marboxil. The granules must be reconstituted in about 15 to 20 mL of room temperature drinking water. The inactive ingredients are: hypromellose, maltitol, mannitol, povidone K25, silicon dioxide, sodium chloride, strawberry flavor, sucralose, and talc.
XOFLUZA for oral suspension (bottles) is supplied as white to light yellow granules in an amber glass bottle. Each bottle contains 40 mg (nominal) of baloxavir marboxil. The granules must be reconstituted with 20 mL of drinking water or sterile water to yield a 2 mg/mL greyish white, white to light yellow opaque suspension with strawberry flavor. The inactive ingredients are: hypromellose, maltitol, mannitol, povidone K25, silicon dioxide, sodium chloride, strawberry flavor, sucralose, and talc.
CLINICAL PHARMACOLOGY
Mechanism of Action
Baloxavir marboxil is an antiviral drug with activity against influenza virus [see Microbiology (12.4) ].
Pharmacodynamics
Cardiac Electrophysiology
At twice the expected exposure from recommended dosing, XOFLUZA did not prolong the QTc interval.
Exposure-Response Relationships
In patients 5 years of age and older, when XOFLUZA is dosed by weight as recommended, a flat baloxavir exposure-response (time to alleviation of influenza symptoms) relationship has been observed.
Pharmacokinetics
Baloxavir marboxil is a prodrug that is almost completely converted to its active metabolite, baloxavir, following oral administration.
Baloxavir pharmacokinetic parameters are presented for healthy adults and adolescents as the mean [% coefficient of variation (%CV)], unless otherwise specified, in Table 5 . Absorption, distribution, metabolism, and elimination data for XOFLUZA is presented in Table 6 .
| Pharmacokinetic Parameters of Plasma Baloxavir in Adults and Adolescents Trial T0831 summary data, mean (%CV) | XOFLUZA dose 40 mg | XOFLUZA dose 80 mg |
|---|---|---|
| AUC (ng∙hr/mL) | 5520 (46.3%) | 6930 (48.6%) |
| C max (ng/mL) | 68.9 (44.9%) | 82.5 (43.0%) |
| C 24 (ng/mL) | 50.9 (45.8%) | 62.6 (45.9%) |
| C 72 (ng/mL) | 24.2 (45.5%) | 30.8 (47.0%) |
| Absorption | |
| T max (hr) Median | 4 |
| Effect of food (relative to fasting) Meal: approximately 400 to 500 kcal including 150 kcal from fat | C max : ↓48%, AUC 0-inf : ↓36% |
| Distribution | |
| % bound to human serum proteins in vitro | 92.9–93.9 |
| Ratio of blood cell to blood | 48.5%–54.4% |
| Volume of distribution (V/F, L) Geometric mean (geometric CV%) | 1180 (20.8%) |
| Elimination | |
| Clearance (CL/F, L/hr) | 10.3 (22.5%) |
| Apparent terminal elimination half-life (hr) | 79.1 (22.4%) |
| Metabolism | |
| Metabolic pathways | Primary: UGT1A3 Secondary: CYP3A4 |
| Excretion | |
| % of dose excreted Ratio of radioactivity to radio-labeled baloxavir marboxil dose in mass balance study | Urine: 14.7 (total radioactivity); 3.3 (baloxavir) Feces: 80.1 (total radioactivity) |
No clinically significant differences in the pharmacokinetics of baloxavir were observed based on age, sex, presence of risk factors for complicated influenza, creatinine clearance (CrCl: 50 mL/min and above), or moderate hepatic impairment (Child-Pugh class B). The effect of severe renal or hepatic impairment on baloxavir pharmacokinetics has not been evaluated.
Pediatrics (5 to < 12 Years of Age)
Mean (CV%) baloxavir pharmacokinetics in pediatric subjects 5 to < 12 years of age are described in Table 7 . Following the approved recommended dosage, baloxavir exposures are similar in pediatric subjects (5 to < 12 years of age) compared to adult and adolescent subjects.
| Pharmacokinetic Parameters of Plasma Baloxavir in Pediatrics Trial CP40563 summary data, mean (%CV); patients not receiving the recommended dose (n=3) were excluded | XOFLUZA Dose for Subjects Weighing < 20 kg | XOFLUZA Dose for Subjects Weighing ≥ 20 kg |
|---|---|---|
| (n=8) | (n=55) | |
| 2 mg/kg | 40 mg | |
| AUC inf (ng.h/mL) | 5830 (48.5) | 4360 (48.9) |
| C max (ng/mL) | 148 (48.7) | 81.1 (44.0) |
| T max (h) Median (range) | 3.5 (2-5.5) | 4.5 (2-23.5) |
| C24 (ng/mL) | 77.9 (49.2) | 52.4 (43.2) |
| C72 (ng/mL) | 19.3 (49.7) | 18.0 (50.9) |
Body Weight
Baloxavir exposure decreases as body weight increases. No clinically significant difference in exposure was observed between body weight groups in adult and pediatric subjects following the approved recommended dosage.
Race/Ethnicity
Based on a population pharmacokinetic analysis, baloxavir exposure is approximately 35% lower in non-Asians as compared to Asians; this difference is not considered clinically significant when the recommended dose was administered.
Drug Interaction Studies
Clinical Studies
No clinically significant changes in the pharmacokinetics of baloxavir marboxil and its active metabolite, baloxavir, were observed when coadministered with itraconazole (combined strong CYP3A and P-gp inhibitor), probenecid (UGT inhibitor), or oseltamivir.
No clinically significant changes in the pharmacokinetics of the following drugs were observed when coadministered with baloxavir marboxil: midazolam (CYP3A4 substrate), digoxin (P-gp substrate), rosuvastatin (BCRP substrate), or oseltamivir.
Animal Studies
Polyvalent Cations: In monkeys, a 48% to 63% decrease in baloxavir exposure was observed when XOFLUZA was coadministered with calcium, aluminum, magnesium, or iron. No study has been conducted in humans.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Both baloxavir marboxil and baloxavir do not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2D6 and do not induce CYP1A2, CYP2B6, or CYP3A4.
Uridine diphosphate (UDP)-glucuronosyl transferase (UGT) Enzymes: Both baloxavir marboxil and baloxavir do not inhibit UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9, UGT2B7, or UGT2B15.
Transporter Systems: Both baloxavir marboxil and baloxavir are substrates of P-glycoprotein (P-gp). Baloxavir does not inhibit organic anion-transporting polypeptides (OATP) 1B1, OATP1B3, organic cation transporter (OCT) 1, OCT2, organic anion transporter (OAT) 1, OAT3, multidrug and toxin extrusion (MATE) 1, or MATE2K.
Microbiology
Mechanism of Action
Baloxavir marboxil is a prodrug that is converted by hydrolysis to baloxavir, the active form that exerts anti-influenza virus activity. Baloxavir inhibits the endonuclease activity of the polymerase acidic (PA) protein, an influenza virus-specific enzyme in the viral RNA polymerase complex required for viral gene transcription, resulting in inhibition of influenza virus replication. The 50% inhibitory concentration (IC 50 ) values of baloxavir ranged from 1.4 to 3.1 nM (n=4) for influenza A viruses and 4.5 to 8.9 nM (n=3) for influenza B viruses in a PA endonuclease assay. Viruses with reduced susceptibility to baloxavir have amino acid substitutions in the PA protein.
Antiviral Activity
The antiviral activity of baloxavir against laboratory strains and clinical isolates of influenza A and B viruses was determined in an MDCK cell-based plaque reduction assay. The median 50% effective concentration (EC 50 ) values of baloxavir were 0.73 nM (n=31; range: 0.20–1.85 nM) for subtype A/H1N1 strains, 0.83 nM (n=33; range: 0.35–2.63 nM) for subtype A/H3N2 strains, and 5.97 nM (n=30; range: 2.67–14.23 nM) for type B strains. In an MDCK cell-based virus titer reduction assay, the 90% effective concentration (EC 90 ) values of baloxavir against avian subtypes A/H5N1 and A/H7N9 were in the range of 0.80 to 3.16 nM. The relationship between antiviral activity in cell culture and clinical response to treatment in humans has not been established.
Resistance
Cell Culture
Influenza A virus isolates with reduced susceptibility to baloxavir were selected by serial passage of virus in cell culture in the presence of increasing concentrations of baloxavir. Reduced susceptibility of influenza A virus to baloxavir was conferred by amino acid substitutions I38T (A/H1N1 and A/H3N2), E198K (A/H1N1) and E199G (A/H3N2) in the PA protein of the viral RNA polymerase complex. An E18G (A/H1N1) substitution in the PA protein, selected by a baloxavir analog, also conferred reduced susceptibility to baloxavir.
Clinical Studies
Treatment-emergent substitutions were identified in influenza A and B viruses in clinical studies. Substitutions associated with a >3-fold reduction in susceptibility to baloxavir are shown in Table 8 .
| Influenza Type/Subtype | A/H1N1 | A/H3N2 | B |
|---|---|---|---|
| Amino Acid Substitution | E23G/K/R, A37T, I38F/N/S/T | E23G/K, A37T, I38M/T, E199G | T20K, I38T |
Clinical studies in adult and adolescent subjects ≥ 12 years of age:
In adult and adolescent subjects who had a confirmed influenza virus infection, the overall frequencies of treatment-emergent amino acid substitutions associated with reduced susceptibility to baloxavir were 5% (6/134), 11% (53/485), and 1% (2/224) in influenza A/H1N1, A/H3N2, and B virus infections, respectively, in pooled data from Trials T0821, T0831, and T0832 [see Clinical Studies (14) ]. In Trial T0834, of 303 subjects ≥ 12 years of age who received XOFLUZA post-exposure prophylaxis, 32 were viral RNA-positive post-baseline, including 17 subjects who were evaluated for resistance. Of these 17 subjects, influenza virus with substitutions associated with reduced susceptibility to baloxavir was identified in 4/4 subjects who developed clinical influenza (as described for the primary endpoint) and 6/13 other subjects evaluated who did not meet the primary endpoint definition for clinical influenza [see Clinical Studies (14) ] .
Clinical studies in pediatric subjects 5 to < 12 years of age:
Selection of influenza viruses with treatment-emergent amino acid substitutions associated with reduced susceptibility to baloxavir has occurred at higher frequencies in pediatric subjects 5 to <12 years of age compared to subjects ≥ 12 years of age. Such viruses were detected with overall frequencies of 17% (2/12), 18% (17/93), and 0% (0/13) in influenza A/H1N1, A/H3N2, and B virus infections, respectively, in pooled data from 4 pediatric treatment trials in subjects 5 to < 12 years of age.
In Trial T0834, of a subgroup of 57 subjects 5 to < 12 years of age who received XOFLUZA post-exposure prophylaxis, 12 were viral-RNA positive post-baseline, including 10 subjects who were evaluated for resistance. Of these 10 subjects, influenza virus with substitutions associated with reduced susceptibility to baloxavir was identified in 2/2 subjects who developed clinical influenza (as described for the primary endpoint) and 1/8 other subjects who did not meet the primary endpoint definition for clinical influenza [see Clinical Studies (14) ].
Clinical studies in pediatrics subjects < 5 years of age:
The highest frequencies of treatment-emergent resistance have been observed in pediatric subjects < 5 years of age. In treatment trials in subjects < 5 years of age, treatment-emergent amino acid substitutions associated with reduced susceptibility to baloxavir occurred in 23% (5/22), 58% (32/55), and 5% (1/19) of influenza A/H1N1, A/H3N2, and B virus infections, respectively, in pooled data from 5 pediatric treatment trials.
Surveillance Studies
Amino acid substitutions associated with reduced susceptibility to baloxavir have also been identified in surveillance studies or in cell culture studies evaluating the impact of resistance substitutions identified in one influenza virus type/subtype on baloxavir susceptibility when introduced into other influenza virus types/subtypes (Table 9 ).
| Influenza Type/Subtype | A/H1N1 | A/H3N2 | B |
|---|---|---|---|
| Amino Acid Substitution | A36V Identified in human surveillance isolates, antiviral treatment unknown , I38L/M Known substitution associated with reduced susceptibility to baloxavir identified in clinical specimens or surveillance isolates but evaluated in the indicated alternative type/subtype by reverse genetics | E23R, A36V, I38F/N/S | I38F/M/N/S |
None of the treatment-emergent substitutions associated with reduced susceptibility to baloxavir were identified in virus from pretreatment respiratory specimens in the clinical studies.
Treatment-emergent resistance has been associated with influenza virus rebound and prolonged virus shedding; however, the impact of prolonged shedding on clinical outcomes and virus transmission potential is currently unknown.
The frequency of baloxavir resistance and the prevalence of such resistant virus may vary seasonally and geographically. Prescribers should consider available information from the U.S. CDC and/or a local health department on current influenza virus drug susceptibility patterns and treatment effects when deciding whether to use XOFLUZA.
Cross-Resistance
Cross-resistance between baloxavir and neuraminidase (NA) inhibitors, or between baloxavir and M2 proton pump inhibitors (adamantanes), is not expected because these drugs target different viral proteins. The NA inhibitor oseltamivir is active against viruses with reduced susceptibility to baloxavir, including A/H1N1 virus with PA substitutions E23K or I38F/T; A/H3N2 virus with PA substitutions E23G/K, A37T, I38M/T, or E199G; and type B virus with the PA substitution I38T. Influenza virus may carry amino acid substitutions in PA that reduce susceptibility to baloxavir and at the same time carry resistance-associated substitutions for NA inhibitors and M2 proton pump inhibitors.
Baloxavir is active against NA inhibitor-resistant strains, including A/H1N1 and A/H5N1 viruses with the NA substitution H275Y (A/H1N1 numbering), A/H3N2 virus with the NA substitutions E119V or R292K, A/H7N9 virus with the NA substitution R292K (A/H3N2 numbering), and type B virus with the NA substitutions R152K or D198E (A/H3N2 numbering). The clinical relevance of phenotypic cross-resistance evaluations has not been established.
Immune Response
Interaction studies with influenza vaccines and baloxavir marboxil have not been conducted.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies have not been performed with baloxavir marboxil.
Mutagenesis
Baloxavir marboxil and the active metabolite, baloxavir, were not mutagenic in in vitro and in in vivo genotoxicity assays, which included bacterial mutation assays in S. typhimurium and E. coli , micronucleus tests with cultured mammalian cells, and in the rodent micronucleus assay.
Impairment of Fertility
In a fertility and early embryonic development study in rats, doses of baloxavir marboxil at 20, 200, or 1,000 mg/kg/day were administered to females for 2 weeks before mating, during mating, and until day 7 of pregnancy. Males were dosed for 4 weeks before mating and throughout mating. There were no effects on fertility, mating performance, or early embryonic development at any dose level, resulting in systemic drug exposure (AUC) approximately 5 times the MRHD.
CLINICAL STUDIES
Treatment of Acute Uncomplicated Influenza—Otherwise Healthy Subjects (12 Years of Age and Older)
Two randomized, controlled, double-blinded clinical trials conducted in two different influenza seasons evaluated efficacy and safety of XOFLUZA in otherwise healthy subjects with acute uncomplicated influenza.
In Trial T0821, a placebo-controlled phase 2 dose-finding trial, a single oral dose of XOFLUZA was compared with placebo in 400 adult subjects 20 to 64 years of age in Japan. All subjects in Trial T0821 were Asian, the majority of subjects were male (62%), and the mean age was 38 years. In this trial, among subjects who received XOFLUZA and had influenza virus typed, influenza A/H1N1 was the predominant strain (63%), followed by influenza B (25%), and influenza A/H3N2 (12%).
In Trial T0831 (NCT02954354), a phase 3, randomized, double-blind, active- and placebo-controlled trial, XOFLUZA was studied in 1,436 otherwise healthy adults and adolescents with signs and symptoms of influenza in the U.S. and Japan. Subjects were 12 to 64 years of age and weighed at least 40 kg. Adults aged 20 to 64 years received weight-based XOFLUZA (subjects who weighed 40 to less than 80 kg received 40 mg and subjects who weighed 80 kg and above received 80 mg) (N=612) or placebo as a single oral dose on day 1 (N=310) or oseltamivir twice a day for 5 days (N=514). Subjects in the XOFLUZA and placebo arms received a placebo for the duration of oseltamivir dosing after XOFLUZA or placebo dosing in that arm. Adolescent subjects 12 to less than 20 years of age received weight-based XOFLUZA or placebo as a single oral dose.
Seventy-eight percent of subjects in Trial T0831 were Asian, 17% were White, and 4% were Black or African American. The mean age was 34 years, and 11% of subjects were less than 20 years of age; 54% of subjects were male and 46% female. In Trial T0831, 1,062 of 1,436 enrolled subjects had influenza confirmed by RT-PCR and were included in the efficacy analysis (XOFLUZA N=455, placebo N=230, or oseltamivir N=377). Among subjects who received XOFLUZA and had influenza virus typed, influenza A/H3N2 was the predominant strain (90%), followed by influenza B (9%), and influenza A/H1N1 (2%).
In both Trials T0821 and T0831, eligible subjects had an axillary temperature of at least 38˚C, at least one moderate or severe respiratory symptom (cough, nasal congestion, or sore throat), and at least one moderate or severe systemic symptom (headache, feverishness or chills, muscle or joint pain, or fatigue), and all were treated within 48 hours of symptom onset. Subjects participating in the trial were required to self-assess their influenza symptoms as "none," "mild," "moderate," or "severe" twice daily. The primary efficacy population was defined as those with a positive rapid influenza diagnostic test (Trial T0821) or positive influenza reverse transcription polymerase chain reaction (RT-PCR) (Trial T0831) at trial entry.
The primary endpoint of both trials, time to alleviation of symptoms, was defined as the time when all seven symptoms (cough, sore throat, nasal congestion, headache, feverishness, myalgia, and fatigue) had been assessed by the subject as none or mild for a duration of at least 21.5 hours.
In both trials, XOFLUZA treatment at the recommended dose resulted in a statistically significant shorter time to alleviation of symptoms compared with placebo in the primary efficacy population (Tables 10 and 11 ).
| XOFLUZA 40 mg (95% CI CI: Confidence interval ) N=100 | Placebo (95% CI) N=100 | |
|---|---|---|
| Adults (20 to 64 Years of Age) | 50 hours XOFLUZA treatment resulted in a statistically significant shorter time to alleviation of symptoms compared to placebo using the Gehan-Breslow's generalized Wilcoxon test (p-value: 0.014, adjusted for multiplicity using the Bonferroni method). The primary analysis using the Cox Proportional Hazards Model did not reach statistical significance (p-value: 0.165). (45, 64) | 78 hours (68, 89) |
| XOFLUZA 40 mg or 80 mg (95% CI CI: Confidence interval ) N=455 | Placebo (95% CI) N=230 | |
|---|---|---|
| Subjects (≥ 12 Years of Age) | 54 hours XOFLUZA treatment resulted in a statistically significant shorter time to alleviation of symptoms compared to placebo using the Peto-Prentice's generalized Wilcoxon test (p-value: < 0.001). (50, 59) | 80 hours (73, 87) |
In Trial T0831, there was no difference in the time to alleviation of symptoms between subjects (age ≥ 20 years) who received XOFLUZA (54 hours) and those who received oseltamivir (54 hours). For adolescent subjects (12 to 17 years of age) in Trial T0831, the median time to alleviation of symptoms for subjects infected with influenza and who received XOFLUZA (N=63) was 54 hours (95% CI of 43, 81) compared to 93 hours (95% CI of 64, 118) in the placebo arm (N=27).
The number of subjects who received XOFLUZA at the recommended dose and who were infected with influenza type B virus was limited, including 24 subjects in Trial T0821 and 38 subjects in Trial T0831. In the influenza B subset in Trial T0821, the median time to alleviation of symptoms in subjects who received 40 mg XOFLUZA was 63 hours (95% CI of 43, 70) compared to 83 hours (95% CI of 58, 93) in subjects who received placebo. In the influenza B subset in Trial T0831, the median time to alleviation of symptoms in subjects who received 40 mg or 80 mg XOFLUZA was 93 hours (95% CI of 53, 135) compared to 77 hours (95% CI of 47, 189) in subjects who received placebo.
Treatment of Acute Uncomplicated Influenza—High Risk Subjects (12 Years of Age and Older)
Trial T0832 (NCT02949011) was a randomized, double-blind, placebo- and active-controlled trial to evaluate the efficacy and safety of a single oral dose of XOFLUZA compared with placebo or oseltamivir in adult and adolescent subjects 12 years of age or older with influenza who were at high risk of developing influenza-related complications.
A total of 2,182 subjects with signs and symptoms of influenza were randomized to receive a single oral dose of 40 mg or 80 mg of XOFLUZA according to body weight (subjects who weighed 40 to less than 80 kg received 40 mg and subjects who weighed 80 kg and above received 80 mg) (N=729), oseltamivir 75 mg twice daily for 5 days (N=725), or placebo (N=728). Twenty-eight percent of subjects were Asian, 59% were White, and 10% were Black or African American. The mean age was 52 years, and 3% of subjects were less than 18 years of age; 43% of subjects were male and 57% female.
High risk factors were based on the Centers for Disease Control and Prevention definition 1 of health factors known to increase the risk of developing serious complications from influenza. The majority of subjects had underlying asthma or chronic lung disease, diabetes, heart disease, morbid obesity, or were 65 years of age or older.
In Trial T0832, 1,158 of the 2,182 enrolled subjects had influenza confirmed by RT-PCR and were included in the efficacy analysis (XOFLUZA N=385, placebo N=385, or oseltamivir N=388). Among subjects in whom only one type/subtype of influenza virus was identified, 50% were infected with subtype A/H3N2, 43% were infected with type B, and 7% were infected with subtype A/H1N1.
Eligible subjects had an axillary temperature of at least 38°C, at least one moderate or severe respiratory symptom (cough, nasal congestion, or sore throat), and at least one moderate or severe systemic symptom (headache, feverishness or chills, muscle or joint pain, or fatigue), and all were treated within 48 hours of symptom onset. Subjects participating in the trial were required to self-assess their influenza symptoms as "none," "mild," "moderate," or "severe" twice daily. A total of 215 subjects (19%) had preexisting symptoms (cough, muscle or joint pain, or fatigue) associated with their underlying high-risk condition that were worsened due to influenza infection. The primary efficacy endpoint was time to improvement of influenza symptoms (cough, sore throat, headache, nasal congestion, feverishness or chills, muscle or joint pain, and fatigue). This endpoint included alleviation of new symptoms and improvement of any preexisting symptoms that had worsened due to influenza. A statistically significant improvement in the primary endpoint was observed for XOFLUZA when compared with placebo (see Table 12 ).
| XOFLUZA 40 mg or 80 mg The dosage of XOFLUZA was based on subject's weight. (95% CI CI: Confidence interval ) N=385 | Placebo (95% CI) N=385 |
|---|---|
| 73 hours XOFLUZA treatment resulted in a significant reduction in Time to Improvement of Influenza Symptoms compared to placebo using Peto-Prentice's generalized Wilcoxon test (p-value: < 0.001). (67, 85) | 102 hours (93, 113) |
There was no statistically significant difference in the median time to improvement of influenza symptoms in the subjects who received XOFLUZA (73 hours) and those who received oseltamivir (81 hours). The median time to improvement of influenza symptoms in the limited number of adolescent subjects aged 12 to 17 years infected with influenza virus was similar for subjects who received XOFLUZA (188 hours) or placebo (191 hours) (N=13 and N=12, respectively).
For subjects infected with type B virus, the median time to improvement of influenza symptoms was 75 hours in the XOFLUZA group (95% CI of 67, 90) compared to 101 hours in the placebo group (95% CI of 83, 116).
Treatment of Acute Uncomplicated Influenza—Otherwise Healthy and High-Risk Pediatric Subjects (5 to < 12 Years of Age)
Trial CP40563 (NCT03629184) was a randomized, double-blind, multicenter, active-controlled trial, designed to evaluate the safety, efficacy, and pharmacokinetics of a single oral dose of XOFLUZA compared with oseltamivir in otherwise healthy pediatric subjects (including subjects aged 5 to < 12 years of age) with influenza-like symptoms. Eligible subjects had a tympanic temperature of at least 38°C and at least one respiratory symptom of either cough or nasal congestion.
A total of 118 subjects 5 to less than 12 years of age were randomized and received a single one-time oral dose of XOFLUZA (N=79) based on body weight (2 mg/kg for subjects weighing < 20 kg or 40 mg for subjects weighing ≥ 20 kg) or oseltamivir (N=39) for 5 days (dose based on body weight). Subjects at high risk of developing complications associated with influenza were included in the trial [16% (19/118)]. The primary objective was to compare the safety of a single one-time dose of XOFLUZA with 5 days of oseltamivir administered twice daily. The secondary efficacy endpoint included time to alleviation of influenza signs and symptoms, which was defined as the time when all of the following were met for at least 21.5 hours: cough and nasal symptoms were assessed by the caregiver as no problem or minor problem, subject was able to return to normal daily activity, and subject was afebrile (temperature ≤ 37.2°C). However, the trial was not powered to detect statistically significant differences in this secondary endpoint.
Of the 118 randomized subjects 5 to less than 12 years of age in Trial CP40563, 94 subjects had influenza confirmed by RT-PCR at baseline or during the trial; 89% percent of subjects were White, 3% Black or African American and 8% Other/unknown/multiple races. The mean age was 8 years [SD=1.97]; 56% of subjects were female and 44% male. The predominant influenza virus strain in this trial was the A/H3N2 subtype (67%), followed by A/H1N1 (20%) and type B (9%).
The median time to alleviation of influenza signs and symptoms was 138 hours in the XOFLUZA arm (95% CI of 117, 163) and 126 hours in the oseltamivir arm (95% CI of 96, 166).
Post-Exposure Prophylaxis of Influenza (5 Years of Age and Older)
Trial T0834 was a phase 3, randomized, double-blind, multicenter, placebo-controlled trial designed to evaluate the efficacy of a single oral dose of XOFLUZA compared with placebo in the prevention of influenza in subjects who were household contacts of influenza-infected patients in Japan. Influenza-infected index patients were required to have onset of symptoms for ≤ 48 hours, and subjects (household contacts) were required to have lived with the influenza-infected index patient for ≥ 48 hours.
A total of 715 subjects (XOFLUZA N=360, placebo N=355) 5 years of age and older were randomized and received a single oral dose of XOFLUZA according to body weight and age, or placebo, on Day 1. Subjects received a single dose of XOFLUZA according to body weight. The primary efficacy endpoint was the proportion of household subjects who were infected with influenza virus and presented with fever and at least one respiratory symptom from day 1 to day 10. Influenza infection was confirmed by RT-PCR, fever was defined as a body temperature (axillary) ≥ 37.5°C, and respiratory symptoms were defined as having a symptom of "cough" or "nasal discharge/nasal congestion" with a severity of moderate or severe as assessed by the subject.
The mean age of subjects that were ≥ 5 years of age in Trial T0834 was 35 years; 108 (15%) were 5 to < 12 years, 33 (5%) were ≥ 12 to < 18 years of age, 551 (77%) were ≥ 18 to < 65 years of age, and 23 (3%) were ≥ 65 years of age. All subjects were Asian, 80% were female, and 20% were male.
In subjects that were 5 years of age and older, there was a statistically significant reduction in the proportion of household contacts (subjects) with laboratory-confirmed clinical influenza from 13% in the placebo group to 2% in the XOFLUZA group (see Table 13 ).
| XOFLUZA (95% CI CI: Confidence interval (%) XOFLUZA treatment resulted in a significant reduction in the risk ratio of patients who were infected with influenza virus and presented with fever compared to placebo using modified Poisson regression for a binary response (p-value: < 0.0001). ) N=360 | Placebo (95% CI) N=355 |
|---|---|
| 6 (2%) | 47 (13%) |
| (1%, 4%) | (10%, 17%) |
In the 108 pediatric subjects 5 to less than 12 years of age enrolled in Trial T0834, 57 subjects received XOFLUZA and 51 received placebo. In this age group, the proportion of subjects with laboratory-confirmed clinical influenza was 4% in the XOFLUZA group and 14% in the placebo group.
HOW SUPPLIED/STORAGE AND HANDLING
XOFLUZA is supplied as tablets (40 mg and 80 mg), granules (30 mg and 40 mg) that are reconstituted into a for oral suspension (packets) and as granules that are reconstituted into a for oral suspension (bottles) [40 mg/20 mL (2 mg/mL)] . The single oral dose to be administered depends on body weight [see Dosage and Administration (2.2) ].
XOFLUZA Tablets
How Supplied
- 40 mg white to light yellow, oblong-shaped, film-coated tablets debossed with "BXM40" on one side available as:
- 1 × 40 mg tablet per blister card in secondary packaging: NDC 50242-860-01
- 80 mg white to light yellow, oblong shaped, film-coated tablets debossed with "BXM80" on one side available as:
- 1 × 80 mg tablet per blister card in secondary packaging: NDC 50242-877-01
Storage: Store tablets in their blister package at 20°C to 25°C (68°F to 77°F); excursions are permitted between 15°C and 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
XOFLUZA for Oral Suspension (Packets)
How Supplied: XOFLUZA for oral suspension (packets) are supplied as granules that are white to light yellow in packets. The granules are reconstituted in about 15 to 20 mL of room temperature drinking water to form an oral suspension. Each carton contains 1 packet available as:
- 1 × 30 mg packet: NDC 50242-599-01
- 1 × 40 mg packet: NDC 50242-617-01
There is no preservative.
Storage: Store granules (packets) at 20°C to 25°C (68°F to 77°F) and keep in the original package; excursions are permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
XOFLUZA for Oral Suspension (Bottles)
How Supplied: XOFLUZA for oral suspension, 40 mg/20 mL (2 mg/mL) are supplied as white to light yellow granules in an amber glass bottle with a child-resistant cap. When reconstituted with drinking water or sterile water, the usable volume of the for oral suspension is 20 mL, equivalent to 40 mg of baloxavir marboxil. XOFLUZA for oral suspension is available as:
- 40 mg/20 mL (2 mg/mL) for oral suspension: NDC 50242-583-01
There is no preservative. Must administer within 10 hours after reconstitution.
Storage: Store granules (bottles) at 20°C to 25°C (68°F to 77°F) and keep in the original bottle; excursions are permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature] .
Store reconstituted suspension no longer than 10 hours at 20°C to 25°C (68°F to 77°F) when reconstituted with drinking water or sterile water. The suspension must be discarded if not used within 10 hours of preparation or if suspension has been stored above 25°C (77°F).
INSTRUCTIONS FOR USE XOFLUZA ® (zoh-FLEW-zuh) (baloxavir marboxil) for oral suspension
This Instruction for Use contains information on how to prepare and take or give XOFLUZA for oral suspension in packets.
Read these Instructions for Use before preparing and taking or giving XOFLUZA for oral suspension. These Instructions for Use do not take the place of talking to your or your child's healthcare provider about your or your child's medical condition or treatment.
Important information you need to know before preparing and taking or giving XOFLUZA for oral suspension
- XOFLUZA is prescribed as a single, one-time dose.
- Wash your hands before and after preparing, taking, or giving XOFLUZA for oral suspension.
- XOFLUZA for oral suspension packets must be mixed with 1 tablespoon (about 15 to 20 mL) of room temperature drinking water.
- Take or give XOFLUZA for oral suspension right away after mixing.
- Check the expiration date and the product for damage before use. Do not use if expired or damaged.
- If you are prescribed the 80 mg dose of XOFLUZA for oral suspension, you will need to take two packets of 40 mg XOFLUZA, one at a time, until your full prescribed dose is taken.
- XOFLUZA for oral suspension can be taken by mouth or given through a feeding tube. Follow your healthcare provider's instructions for giving XOFLUZA through a feeding tube.
Your healthcare provider will prescribe XOFLUZA packets based on your or your child's weight. See the dosing chart below for the prescribed dose and number of XOFLUZA packets needed based on your or your child's weight.
| Body weight | Prescribed dose and number of XOFLUZA packets needed |
|---|---|
| 33 lbs (15 kg) to less than 44 lbs (20 kg) | 30 mg (One 30 mg packet) |
| 44 lbs (20 kg) to less than 176 lbs (80 kg) | 40 mg (One 40 mg packet) |
| At least 176 lbs (80 kg) and above | 80 mg (two 40 mg packets) |
Supplies needed to prepare and take or give XOFLUZA for oral suspension:
- your prescribed dose of XOFLUZA packets
- a tablespoon (about 15 to 20 mL) of room temperature drinking water in a container
- an enteral syringe (if giving XOFLUZA for oral suspension through a feeding tube).
| Preparing XOFLUZA for oral suspension | |
| Step 1. Wash and dry your hands. | |
Step 2: Check your dose Your healthcare provider will prescribe:
| 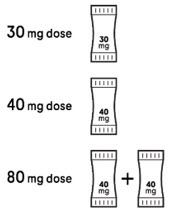 |
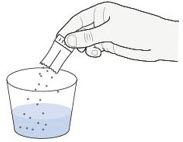
| Step 3. Tap the packet to make sure the granules are on one side of the packet. Open the packet by hand or with scissors. (Figure A ) Do not cut the granules with the scissor. |  |
| Figure A | |
| Step 4. Add the granules to a small container containing 1 tablespoon (about 15 to 20 mL) of room temperature drinking water. (Figure B ) Tap the packet to make sure all granules have been removed. |
|
| Figure B | |
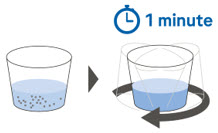
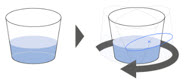
| Step 5. Gently swirl the cup for 1 minute, or until the granules have fully dispersed. (Figure C ) Go to Step 6 for taking XOFLUZA for oral suspension by mouth, or Go to Step 10 for giving XOFLUZA for oral suspension through a feeding tube. |
|
| Figure C | |
| Taking XOFLUZA for oral suspension by mouth | |
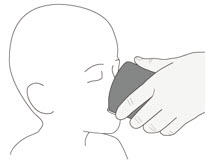
| Step 6. Drink the mixture right away. (Figure D ) |
|
| Figure D | |
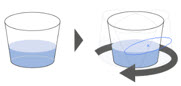
| Step 7. Refill the container with 1 tablespoon (about 15 to 20 mL) of drinking water and swirl to get any remaining granules. (Figure E ) Drink right away. Repeat this step one more time if there are still granules left in the container. If your prescribed dose requires two XOFLUZA packets, repeat Steps 2 through 7 . |
|
| Figure E | |
| Step 8. Wash your hands and all supplies used to take XOFLUZA for oral suspension. (Figure F ) |
|
| Figure F | |
| Step 9. Throw away the empty packet and clean the container with soap and water. | |
| Giving XOFLUZA for oral suspension through a feeding tube | |
| |
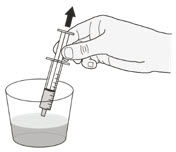
| Step 10 Place the enteral syringe tip right away into the container and slowly pull up the plunger to draw up all the oral suspension. (Figure G ) Do not wait to draw up the oral suspension. If the mixture sits for too long, it may settle to the bottom, and you may not get the full dose. |
|
| Figure G | |
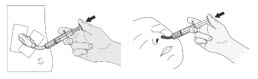
| Step 11 Place the enteral syringe tip into the feeding tube. Slowly push the plunger all the way down to give XOFLUZA. (Figure H ) |
|
| Figure H | |
| Step 12 Refill the container with 1 tablespoon (about 15 to 20 mL) of drinking water and swirl to get any remaining granules left in the container. (Figure I ) Use this water to flush the feeding tube. Repeat this step one more time if there are still granules left in the container. If your prescribed dose requires 2 XOFLUZA packets, repeat Steps 2 through 5 to prepare the XOFLUZA for oral suspension, then repeat Steps 10 through 12. |
|
| Figure I | |
| Step 13 Wash your hands with soap and water. Follow the manufacturer's instructions to clean your enteral syringe. | |
| Storing XOFLUZA for oral suspension in packets | |
| |
Distributed by: Genentech USA, Inc. , A Member of the Roche Group 1 DNA Way, South San Francisco, CA 94080-4990 XOFLUZA® is a registered trademark of Genentech, Inc. ©2025 Genentech USA, Inc. This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 05/2025
Mechanism of Action
Baloxavir marboxil is an antiviral drug with activity against influenza virus [see Microbiology (12.4) ].