Get your patient on Xpovio (Selinexor)
Xpovio prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Xpovio patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- Multiple Myeloma in Combination with Bortezomib and Dexamethasone (XVd): Recommended dosage of XPOVIO is 100 mg taken orally once weekly in combination with bortezomib and dexamethasone (2.1 ).
- Multiple Myeloma in Combination with Dexamethasone (Xd): Recommended dosage of XPOVIO is 80 mg taken orally on Days 1 and 3 of each week in combination with dexamethasone (2.1 ).
- DLBCL: Recommended dosage of XPOVIO is 60 mg taken orally on Days 1 and 3 of each week (2.2 ).
- See Full Prescribing Information for dosage in patients with severe hepatic impairment (2.6 , 8.6 ).
Recommended Dosage for Multiple Myeloma
In Combination with Bortezomib and Dexamethasone (XVd)
The recommended dosage of XPOVIO is 100 mg taken orally once weekly on Day 1 of each week until disease progression or unacceptable toxicity in combination with:
- Bortezomib 1.3 mg/m 2 administered subcutaneously once weekly on Day 1 of each week for 4 weeks followed by 1 week off.
- Dexamethasone 20 mg taken orally twice weekly on Days 1 and 2 of each week.
Refer to Clinical Studies (14.1 ) and the prescribing information of bortezomib and dexamethasone for additional dosing information.
In Combination with Dexamethasone (Xd)
The recommended dosage of XPOVIO is 80 mg taken orally on Days 1 and 3 of each week until disease progression or unacceptable toxicity in combination with dexamethasone 20 mg taken orally with each dose of XPOVIO on Days 1 and 3 of each week.
For additional information regarding the administration of dexamethasone, refer to its prescribing information.
Recommended Dosage for Diffuse Large B-Cell Lymphoma
The recommended dosage of XPOVIO is 60 mg taken orally on Days 1 and 3 of each week until disease progression or unacceptable toxicity.
Recommended Monitoring for Safety
Monitor complete blood count (CBC) with differential, standard blood chemistries, body weight, nutritional status, and volume status at baseline and during treatment as clinically indicated. Monitor more frequently during the first three months of treatment [see Warning and Precautions (5.1 , 5.2 , 5.3 , and 5.4 )] . Assess the need for dosage modifications of XPOVIO for adverse reactions [see Dosage and Administration (2.5 )] .
Recommended Concomitant Treatments
Advise patients to maintain adequate fluid and caloric intake throughout treatment. Consider intravenous hydration for patients at risk of dehydration [see Warnings and Precautions (5.3 , 5.4 )] .
Provide prophylactic antiemetics. Administer a 5-HT3 receptor antagonist and other anti-nausea agents prior to and during treatment with XPOVIO [see Warnings and Precautions (5.3 )] .
Dosage Modifications for Adverse Reactions
Recommended XPOVIO dosage reduction steps are presented in Table 1 .
| Recommended Starting Dosage | Multiple Myeloma In Combination with Bortezomib and Dexamethasone (XVd) | Multiple Myeloma In Combination with Dexamethasone (Xd) | Diffuse Large B-Cell Lymphoma |
| 100 mg once weekly | 80 mg Days 1 and 3 of each week (160 mg total per week) | 60 mg Days 1 and 3 of each week (120 mg total per week) | |
| First Reduction | 80 mg once weekly | 100 mg once weekly | 40 mg Days 1 and 3 of each week (80 mg total per week) |
| Second Reduction | 60 mg once weekly | 80 mg once weekly | 60 mg once weekly |
| Third Reduction | 40 mg once weekly | 60 mg once weekly | 40 mg once weekly |
| Fourth Reduction | Permanently discontinue | Permanently discontinue | Permanently discontinue |
Recommended dosage modifications for hematologic adverse reactions in patients with multiple myeloma and DLBCL are presented in Table 2 and Table 3 , respectively. Recommended dosage modifications for nonhematologic adverse reactions are presented in Table 4 .
| Adverse Reaction | Occurrence | Action |
| Thrombocytopenia [see Warning and Precautions (5.1 )] | ||
| Platelet count 25,000 to less than 75,000/mcL | Any |
|
| Platelet count 25,000 to less than 75,000/mcL with concurrent bleeding | Any |
|
| Platelet count less than 25,000/mcL | Any |
|
| Adverse Reaction | Occurrence | Action |
| Neutropenia [see Warning and Precautions (5.2 )] | ||
| Absolute neutrophil count of 0.5 to 1 x 10 9 /L without fever | Any |
|
| Absolute neutrophil count less than 0.5 x 10 9 /L OR febrile neutropenia | Any |
|
| Anemia | ||
| Hemoglobin less than 8 g/dL | Any |
|
| Life-threatening consequences | Any |
|
| Adverse Reaction | Occurrence | Action |
| Thrombocytopenia [see Warning and Precautions (5.1 )] | ||
| Platelet count 50,000 to less than 75,000/mcL | Any |
|
| Platelet count 25,000 to less than 50,000/mcL without bleeding | 1st |
|
| Platelet count 25,000 to less than 50,000/mcL with concurrent bleeding | Any |
|
| Platelet count less than 25,000/mcL | Any |
|
| Neutropenia [see Warning and Precautions (5.2 )] | ||
| Absolute neutrophil count of 0.5 to less than 1 x 10 9 /L without fever | 1st occurrence |
|
| Recurrence |
| |
| Absolute neutrophil count less than 0.5 x 10 9 /L OR Febrile neutropenia | Any |
|
| Anemia | ||
| Hemoglobin less than 8 g/dL | Any |
|
| Life-threatening consequences | Any |
|
| Adverse Reaction | Occurrence | Action |
| Nausea and Vomiting [see Warning and Precautions (5.3 )] | ||
| Grade 1 or 2 nausea (oral intake decreased without significant weight loss, dehydration or malnutrition) OR Grade 1 or 2 vomiting (5 or fewer episodes per day) | Any |
|
| Grade 3 nausea (inadequate oral caloric or fluid intake) OR Grade 3 or higher vomiting (6 or more episodes per day) | Any |
|
| Diarrhea [see Warning and Precautions (5.3 )] | ||
| Grade 2 (increase of 4 to 6 stools per day over baseline) | 1 st |
|
| 2 nd and subsequent |
| |
| Grade 3 or higher (increase of 7 stools or more per day over baseline; hospitalization indicated) | Any |
|
| Weight Loss and Anorexia [see Warning and Precautions (5.3 )] | ||
| Weight loss of 10% to less than 20% OR Anorexia associated with significant weight loss or malnutrition | Any |
|
| Hyponatremia [see Warning and Precautions (5.4 )] | ||
| Sodium level 130 mmol/L or less | Any |
|
| Fatigue | ||
| Grade 2 lasting greater than 7 days OR Grade 3 | Any |
|
| Ocular Toxicity [see Warning and Precautions (5.8 )] | ||
| Grade 2, excluding cataract | Any |
|
| Grade ≥3, excluding cataract | Any |
|
| Other Non-Hematologic Adverse Reactions [see Warning and Precautions (5.6 )] | ||
| Grade 3 or 4 | Any |
|
Starting Dosage and Dosage Modifications for Adverse Reactions in Patients with Severe Hepatic Impairment
For patients with severe hepatic impairment, defined by NCI-ODWG (National Cancer Institute Organ Dysfunction Working Group) criteria as total bilirubin >3x upper limit of normal with any level of AST, use the starting dosage of XPOVIO as shown in Table 5 [see Use in Specific Populations (8.6 ) and Clinical Pharmacology (12.3 )]. See Table 6 for dosage modifications for adverse reactions in patients with severe hepatic impairment.
AST = aspartate aminotransferase; ULN = upper limit of normal | |||
| Total Bilirubin Levels | XPOVIO Dosage | ||
| Multiple Myeloma in Combination with Bortezomib and Dexamethasone (XVd) | Multiple Myeloma in Combination with Dexamethasone (Xd) | Diffuse Large B Cell Lymphoma | |
| >3x ULN (and any AST) | 80 mg once weekly | 100 mg once weekly | 40 mg Days 1 and 3 of each week (80 mg total per week) |
| Multiple Myeloma In Combination with Bortezomib and Dexamethasone (XVd) | Multiple Myeloma In Combination with Dexamethasone (Xd) | Diffuse Large B-Cell Lymphoma | |
| Recommended Starting Dosage | 80 mg once weekly | 100 mg once weekly | 40 mg Days 1 and 3 of each week (80 mg total per week) |
| First Reduction | 60 mg once weekly | 80 mg once weekly | 60 mg once weekly |
| Second Reduction | 40 mg once weekly | 60 mg once weekly | 40 mg once weekly |
| Third Reduction | Permanently discontinue | Permanently discontinue | Permanently discontinue |
Administration
Each XPOVIO dose should be taken at approximately the same time of day and each tablet should be swallowed whole with water. Do not break, chew, crush, or divide the tablets.
If a dose of XPOVIO is missed or delayed, instruct patients to take their next dose at the next regularly scheduled time.
If a patient vomits a dose of XPOVIO, the patient should not repeat the dose and the patient should take the next dose on the next regularly scheduled day.

By using PrescriberAI, you agree to the AI Terms of Use.
Xpovio prescribing information
Dosage and Administration, Starting Dosage and Dosage Modifications for Adverse Reactions in Patients with Severe Hepatic Impairment (2.6 ) 9/2025
INDICATIONS AND USAGE
XPOVIO is a nuclear export inhibitor indicated:
- In combination with bortezomib and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least one prior therapy (1.1 ).
- In combination with dexamethasone for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody (1.1 ).
- For the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least 2 lines of systemic therapy. This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s) (1.2 ).
Multiple Myeloma
- XPOVIO in combination with bortezomib and dexamethasone is indicated for the treatment of adult patients with multiple myeloma who have received at least one prior therapy.
- XPOVIO in combination with dexamethasone is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody.
Diffuse Large B-Cell Lymphoma
XPOVIO is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least 2 lines of systemic therapy.
This indication is approved under accelerated approval based on response rate [see Clinical Studies (14.2 )] . Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
DOSAGE AND ADMINISTRATION
- Multiple Myeloma in Combination with Bortezomib and Dexamethasone (XVd): Recommended dosage of XPOVIO is 100 mg taken orally once weekly in combination with bortezomib and dexamethasone (2.1 ).
- Multiple Myeloma in Combination with Dexamethasone (Xd): Recommended dosage of XPOVIO is 80 mg taken orally on Days 1 and 3 of each week in combination with dexamethasone (2.1 ).
- DLBCL: Recommended dosage of XPOVIO is 60 mg taken orally on Days 1 and 3 of each week (2.2 ).
- See Full Prescribing Information for dosage in patients with severe hepatic impairment (2.6 , 8.6 ).
Recommended Dosage for Multiple Myeloma
In Combination with Bortezomib and Dexamethasone (XVd)
The recommended dosage of XPOVIO is 100 mg taken orally once weekly on Day 1 of each week until disease progression or unacceptable toxicity in combination with:
- Bortezomib 1.3 mg/m 2 administered subcutaneously once weekly on Day 1 of each week for 4 weeks followed by 1 week off.
- Dexamethasone 20 mg taken orally twice weekly on Days 1 and 2 of each week.
Refer to Clinical Studies (14.1 ) and the prescribing information of bortezomib and dexamethasone for additional dosing information.
In Combination with Dexamethasone (Xd)
The recommended dosage of XPOVIO is 80 mg taken orally on Days 1 and 3 of each week until disease progression or unacceptable toxicity in combination with dexamethasone 20 mg taken orally with each dose of XPOVIO on Days 1 and 3 of each week.
For additional information regarding the administration of dexamethasone, refer to its prescribing information.
Recommended Dosage for Diffuse Large B-Cell Lymphoma
The recommended dosage of XPOVIO is 60 mg taken orally on Days 1 and 3 of each week until disease progression or unacceptable toxicity.
Recommended Monitoring for Safety
Monitor complete blood count (CBC) with differential, standard blood chemistries, body weight, nutritional status, and volume status at baseline and during treatment as clinically indicated. Monitor more frequently during the first three months of treatment [see Warning and Precautions (5.1 , 5.2 , 5.3 , and 5.4 )] . Assess the need for dosage modifications of XPOVIO for adverse reactions [see Dosage and Administration (2.5 )] .
Recommended Concomitant Treatments
Advise patients to maintain adequate fluid and caloric intake throughout treatment. Consider intravenous hydration for patients at risk of dehydration [see Warnings and Precautions (5.3 , 5.4 )] .
Provide prophylactic antiemetics. Administer a 5-HT3 receptor antagonist and other anti-nausea agents prior to and during treatment with XPOVIO [see Warnings and Precautions (5.3 )] .
Dosage Modifications for Adverse Reactions
Recommended XPOVIO dosage reduction steps are presented in Table 1 .
| Recommended Starting Dosage | Multiple Myeloma In Combination with Bortezomib and Dexamethasone (XVd) | Multiple Myeloma In Combination with Dexamethasone (Xd) | Diffuse Large B-Cell Lymphoma |
| 100 mg once weekly | 80 mg Days 1 and 3 of each week (160 mg total per week) | 60 mg Days 1 and 3 of each week (120 mg total per week) | |
| First Reduction | 80 mg once weekly | 100 mg once weekly | 40 mg Days 1 and 3 of each week (80 mg total per week) |
| Second Reduction | 60 mg once weekly | 80 mg once weekly | 60 mg once weekly |
| Third Reduction | 40 mg once weekly | 60 mg once weekly | 40 mg once weekly |
| Fourth Reduction | Permanently discontinue | Permanently discontinue | Permanently discontinue |
Recommended dosage modifications for hematologic adverse reactions in patients with multiple myeloma and DLBCL are presented in Table 2 and Table 3 , respectively. Recommended dosage modifications for nonhematologic adverse reactions are presented in Table 4 .
| Adverse Reaction | Occurrence | Action |
| Thrombocytopenia [see Warning and Precautions (5.1 )] | ||
| Platelet count 25,000 to less than 75,000/mcL | Any |
|
| Platelet count 25,000 to less than 75,000/mcL with concurrent bleeding | Any |
|
| Platelet count less than 25,000/mcL | Any |
|
| Adverse Reaction | Occurrence | Action |
| Neutropenia [see Warning and Precautions (5.2 )] | ||
| Absolute neutrophil count of 0.5 to 1 x 10 9 /L without fever | Any |
|
| Absolute neutrophil count less than 0.5 x 10 9 /L OR febrile neutropenia | Any |
|
| Anemia | ||
| Hemoglobin less than 8 g/dL | Any |
|
| Life-threatening consequences | Any |
|
| Adverse Reaction | Occurrence | Action |
| Thrombocytopenia [see Warning and Precautions (5.1 )] | ||
| Platelet count 50,000 to less than 75,000/mcL | Any |
|
| Platelet count 25,000 to less than 50,000/mcL without bleeding | 1st |
|
| Platelet count 25,000 to less than 50,000/mcL with concurrent bleeding | Any |
|
| Platelet count less than 25,000/mcL | Any |
|
| Neutropenia [see Warning and Precautions (5.2 )] | ||
| Absolute neutrophil count of 0.5 to less than 1 x 10 9 /L without fever | 1st occurrence |
|
| Recurrence |
| |
| Absolute neutrophil count less than 0.5 x 10 9 /L OR Febrile neutropenia | Any |
|
| Anemia | ||
| Hemoglobin less than 8 g/dL | Any |
|
| Life-threatening consequences | Any |
|
| Adverse Reaction | Occurrence | Action |
| Nausea and Vomiting [see Warning and Precautions (5.3 )] | ||
| Grade 1 or 2 nausea (oral intake decreased without significant weight loss, dehydration or malnutrition) OR Grade 1 or 2 vomiting (5 or fewer episodes per day) | Any |
|
| Grade 3 nausea (inadequate oral caloric or fluid intake) OR Grade 3 or higher vomiting (6 or more episodes per day) | Any |
|
| Diarrhea [see Warning and Precautions (5.3 )] | ||
| Grade 2 (increase of 4 to 6 stools per day over baseline) | 1 st |
|
| 2 nd and subsequent |
| |
| Grade 3 or higher (increase of 7 stools or more per day over baseline; hospitalization indicated) | Any |
|
| Weight Loss and Anorexia [see Warning and Precautions (5.3 )] | ||
| Weight loss of 10% to less than 20% OR Anorexia associated with significant weight loss or malnutrition | Any |
|
| Hyponatremia [see Warning and Precautions (5.4 )] | ||
| Sodium level 130 mmol/L or less | Any |
|
| Fatigue | ||
| Grade 2 lasting greater than 7 days OR Grade 3 | Any |
|
| Ocular Toxicity [see Warning and Precautions (5.8 )] | ||
| Grade 2, excluding cataract | Any |
|
| Grade ≥3, excluding cataract | Any |
|
| Other Non-Hematologic Adverse Reactions [see Warning and Precautions (5.6 )] | ||
| Grade 3 or 4 | Any |
|
Starting Dosage and Dosage Modifications for Adverse Reactions in Patients with Severe Hepatic Impairment
For patients with severe hepatic impairment, defined by NCI-ODWG (National Cancer Institute Organ Dysfunction Working Group) criteria as total bilirubin >3x upper limit of normal with any level of AST, use the starting dosage of XPOVIO as shown in Table 5 [see Use in Specific Populations (8.6 ) and Clinical Pharmacology (12.3 )]. See Table 6 for dosage modifications for adverse reactions in patients with severe hepatic impairment.
AST = aspartate aminotransferase; ULN = upper limit of normal | |||
| Total Bilirubin Levels | XPOVIO Dosage | ||
| Multiple Myeloma in Combination with Bortezomib and Dexamethasone (XVd) | Multiple Myeloma in Combination with Dexamethasone (Xd) | Diffuse Large B Cell Lymphoma | |
| >3x ULN (and any AST) | 80 mg once weekly | 100 mg once weekly | 40 mg Days 1 and 3 of each week (80 mg total per week) |
| Multiple Myeloma In Combination with Bortezomib and Dexamethasone (XVd) | Multiple Myeloma In Combination with Dexamethasone (Xd) | Diffuse Large B-Cell Lymphoma | |
| Recommended Starting Dosage | 80 mg once weekly | 100 mg once weekly | 40 mg Days 1 and 3 of each week (80 mg total per week) |
| First Reduction | 60 mg once weekly | 80 mg once weekly | 60 mg once weekly |
| Second Reduction | 40 mg once weekly | 60 mg once weekly | 40 mg once weekly |
| Third Reduction | Permanently discontinue | Permanently discontinue | Permanently discontinue |
Administration
Each XPOVIO dose should be taken at approximately the same time of day and each tablet should be swallowed whole with water. Do not break, chew, crush, or divide the tablets.
If a dose of XPOVIO is missed or delayed, instruct patients to take their next dose at the next regularly scheduled time.
If a patient vomits a dose of XPOVIO, the patient should not repeat the dose and the patient should take the next dose on the next regularly scheduled day.
DOSAGE FORMS AND STRENGTHS
Tablets:
10 mg, blue, round, bi-convex, film-coated tablets with “X10” debossed on one side and nothing on the other side.
20 mg, blue, round, bi-convex, film-coated tablets with “K20” debossed on one side and nothing on the other side.
40 mg tablets, blue, oval, film-coated, debossed on both sides with “X40”.
50 mg tablets, blue, oval, film-coated, debossed on both sides with “X50”.
60 mg tablets, blue, oval, film-coated, debossed on both sides with “X60”.
80 mg tablets, blue, oval, film-coated, debossed on both sides with “X80”.
USE IN SPECIFIC POPULATIONS
Lactation : Advise not to breastfeed (8.2 ).
Pregnancy
Risk Summary
Based on findings in animal studies and its mechanism of action [see Clinical Pharmacology (12.1 )] , XPOVIO can cause fetal harm when administered to a pregnant woman. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of selinexor to pregnant rats during organogenesis resulted in structural abnormalities and alterations to growth at exposures that were below those occurring clinically at the recommended dose (see Data ) . Advise pregnant women of the risks to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal data
In an embryo-fetal development study in pregnant rats, daily oral administration of selinexor at 0, 0.25, 0.75, or 2 mg/kg throughout organogenesis caused incomplete or delayed ossification, skeletal variations, and reduced fetal weight compared with controls at a dose of 0.75 mg/kg (approximately 0.08-fold of human area under the curve [AUC] at the recommended dose). Malformations were observed at 2 mg/kg, including microphthalmia, fetal edema, malpositioned kidney, and persistent truncus arteriosus.
Lactation
Risk Summary
There is no information regarding the presence of selinexor or its metabolites in human milk, or their effects on the breastfed child or milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with XPOVIO and for 1 week after the last dose.
Females and Males of Reproductive Potential
XPOVIO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1 )] .
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating XPOVIO [see Use in Specific Populations (8.1 )] .
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with XPOVIO and for 1 week after the last dose.
Males
Advise males with a female partner of reproductive potential to use effective contraception during treatment with XPOVIO and for 1 week after the last dose.
Infertility
Females and Males
Based on findings in animals, XPOVIO may impair fertility in females and males of reproductive potential [see Nonclinical Toxicology (13.1 )] .
Pediatric Use
The safety and effectiveness of XPOVIO have not been established in pediatric patients.
Geriatric Use
In BOSTON, of the 195 patients with multiple myeloma who received XPOVIO in combination with bortezomib and dexamethasone, 56% were 65 years of age and older, while 17% were 75 years of age and older. No overall differences in effectiveness were observed between these patients and younger patients. When comparing patients 65 years of age and older to younger patients, older patients had a higher incidence of discontinuation due to an adverse reaction (28% vs 13%) and a higher incidence of serious adverse reactions (56% vs 47%).
In STORM, of the 202 patients with multiple myeloma who received XPOVIO, 49% were 65 years of age and older, while 11% were 75 years of age and older. No overall difference in effectiveness was observed in patients over 65 years of age, including patients over 75 years of age, when compared with younger patients. When comparing patients 75 years of age and older to younger patients, older patients had a higher incidence of discontinuation due to an adverse reaction (44% vs 27%), higher incidence of serious adverse reactions (70% vs 58%), and higher incidence of fatal adverse reactions (17% vs 9%).
Among 134 patients with DLBCL who received XPOVIO in SADAL, 61% were 65 years of age and older, while 25% were 75 years of age and older. Clinical studies of XPOVIO in patients with relapsed or refractory DLBCL did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
Hepatic Impairment
No dose adjustment for selinexor is required for mild (total bilirubin ≤1x ULN and AST >1x ULN, or total bilirubin >1.0 to 1.5x ULN and any AST) or moderate (total bilirubin >1.5x to 3.0x ULN, and any AST) hepatic impairment. Reduce the starting dose in patients with severe (total bilirubin >3.0x ULN, and any AST) hepatic impairment [see Dosage and Administration (2.6 ) and Clinical Pharmacology (12.3 )] .
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
- Thrombocytopenia : Monitor platelet counts throughout treatment. Manage with dose interruption and/or reduction and supportive care (2.5 , 5.1 ).
- Neutropenia : Monitor neutrophil counts throughout treatment. Manage with dose interruption and/or reduction and granulocyte colony-stimulating factors (2.5 , 5.2 ).
- Gastrointestinal Toxicity : Nausea, vomiting, diarrhea, anorexia, and weight loss may occur. Provide antiemetic prophylaxis. Manage with dose interruption and/or reduction, antiemetics, and supportive care (2.5 , 5.3 ).
- Hyponatremia : Monitor serum sodium levels throughout treatment.
- Correct for concurrent hyperglycemia and high serum paraprotein levels. Manage with dose interruption, reduction, or discontinuation, and supportive care (2.5 , 5.4 ).
- Serious Infection : Monitor for infection and treat promptly (5.2 , 5.5 ).
- Neurological Toxicity : Advise patients to refrain from driving and engaging in hazardous occupations or activities until neurological toxicity resolves. Optimize hydration status and concomitant medications to avoid dizziness or mental status changes (5.6 ).
- Embryo-Fetal Toxicity : Can cause fetal harm. Advise females of reproductive potential and males with a female partner of reproductive potential, of the potential risk to a fetus and use of effective contraception (5.7 , 8.1 , 8.3 ).
- Cataract : Cataracts may develop or progress. Treatment of cataracts usually requires surgical removal of the cataract (5.8 ).
Thrombocytopenia
XPOVIO can cause life-threatening thrombocytopenia, potentially leading to hemorrhage. Thrombocytopenia is the leading cause of dosage modifications [see Adverse Reactions (6.1 )] .
In patients with multiple myeloma who received XPOVIO 100 mg once weekly (BOSTON, n=195), thrombocytopenia was reported in 92% of patients and severe (Grade 3-4) thrombocytopenia was reported in 43% of patients. The median time to first onset was 22 days for any grade thrombocytopenia and 43 days for Grade 3 or 4 thrombocytopenia. Bleeding occurred in 16% of patients with thrombocytopenia, clinically significant bleeding (Grade ≥3 bleeding) occurred in 4% of patients with thrombocytopenia, and fatal hemorrhage occurred in 2% of patients with thrombocytopenia. Permanent discontinuations of XPOVIO due to thrombocytopenia occurred in 2% of patients.
In patients with multiple myeloma who received XPOVIO 80 mg twice weekly (STORM, n=202), thrombocytopenia was reported as an adverse reaction in 74% of patients and severe (Grade 3-4) thrombocytopenia was reported in 61% of patients. The median time to onset of the first event was 22 days. Bleeding occurred in 23% of patients with thrombocytopenia, clinically significant bleeding occurred in 5% of patients with thrombocytopenia, and fatal hemorrhage occurred in <1% of patients.
In patients with DLBCL who received XPOVIO 60 mg twice weekly (SADAL, n=134), thrombocytopenia developed or worsened in 86% of patients, including Grade 3-4 thrombocytopenia in 49% of patients (Grade 4, 18%). The median time to first onset was 28 days for any grade thrombocytopenia and 33 days for Grade 3 or 4 thrombocytopenia.
Monitor platelet counts at baseline and throughout treatment. Monitor more frequently during the first three months of treatment. Institute platelet transfusion and/or other treatments as clinically indicated. Monitor patients for signs and symptoms of bleeding and evaluate promptly. Interrupt, reduce dose, or permanently discontinue based on severity of adverse reaction [see Dosage and Administration (2.5 )].
Neutropenia
XPOVIO can cause life-threatening neutropenia, potentially increasing the risk of infection [see Adverse Reactions (6.1 )] .
In patients with multiple myeloma who received XPOVIO 100 mg once weekly (BOSTON, n=195), neutropenia was reported in 48% of patients and severe neutropenia (Grade 3-4) was reported in 12% of patients. The median time to onset of the first event was 23 days for any grade neutropenia and 40 days for Grade 3-4 neutropenia. Febrile neutropenia was reported in <1% of patients.
In patients with multiple myeloma who received XPOVIO 80 mg twice weekly (STORM, n=202), neutropenia was reported as an adverse reaction in 34% of patients and severe (Grade 3-4) neutropenia was reported in 21% of patients. The median time to onset of the first event was 25 days. Febrile neutropenia was reported in 3% of patients.
In patients with DLBCL (SADAL, n=134), Grade 3 neutropenia developed in 21% of patients and Grade 4 neutropenia developed in 9% of patients. The median time to first onset of Grade 3 or 4 neutropenia was 32 days. Febrile neutropenia was reported in 3% of patients.
Obtain white blood cell counts with differential at baseline and throughout treatment. Monitor more frequently during the first three months of treatment. Monitor patients for signs and symptoms of concomitant infection and evaluate promptly. Consider supportive measures, including antimicrobials and growth factors (e.g., G-CSF). Interrupt, reduce dose or permanently discontinue based on severity of adverse reaction [see Dosage and Administration (2.5 )].
Gastrointestinal Toxicity
XPOVIO can cause severe gastrointestinal toxicities [see Adverse Reactions (6.1 )] . In patients with DLBCL (n=134), gastrointestinal toxicity occurred in 80% of patients with Grade 3 or 4 in 13%.
Nausea/Vomiting
In patients with multiple myeloma who received XPOVIO once weekly (BOSTON, n=195) with use of antiemetic prophylaxis (88% of patients), nausea was reported in 50% of patients and Grade 3 nausea was reported in 8% of patients. The median time to onset of the first event was 6 days. Vomiting was reported in 21% of patients and Grade 3 vomiting was reported in 4.1% of patients. The median time to onset of the first event was 8 days. Permanent discontinuation due to nausea occurred in 3.1% of patients and due to vomiting occurred in 2.1% of patients.
In patients with multiple myeloma receiving XPOVIO 80 mg twice weekly (STORM, n=202) with use of antiemetic prophylaxis, nausea was reported as an adverse reaction in 72% of patients and Grade 3 nausea occurred in 9%. The median time to first onset of nausea was 3 days. Vomiting was reported in 41% of patients and Grade 3 vomiting occurred in 4% of patients. The median time to first onset of vomiting was 5 days.
In patients with DLBCL (SADAL, n=134) with use of antiemetic prophylaxis, nausea occurred in 57% of patients and Grade 3 nausea occurred in 6% of patients. Vomiting occurred in 28% of patients and Grade 3 vomiting occurred in 1.5% of patients. The median time to first onset was 3 days for nausea and 7 days for vomiting.
Provide prophylactic antiemetics. Administer 5-HT3 receptor antagonists and other anti-nausea agents prior to and during treatment with XPOVIO. Interrupt, reduce dose or permanently discontinue based on severity of adverse reaction [see Dosage and Administration (2.5 )] . Administer intravenous fluids to prevent dehydration and replace electrolytes as clinically indicated.
Diarrhea
In patients with multiple myeloma who received XPOVIO once weekly (BOSTON, n=195), diarrhea was reported in 32% of patients and Grade 3 diarrhea was reported in 6% of patients. The median time to onset of the first event was 50 days. Permanent discontinuation due to diarrhea occurred in 1% of patients.
In patients with multiple myeloma who received XPOVIO 80 mg twice weekly (STORM, n=202), diarrhea was reported as an adverse reaction in 44% of patients and Grade 3 diarrhea occurred in 6% of patients. The median time to onset of diarrhea was 15 days.
In patients with DLBCL (SADAL, n=134), diarrhea occurred in 37% of patients and Grade 3 diarrhea occurred in 3% of patients treated with XPOVIO. The median time to onset of the first event was 12 days.
Interrupt, reduce dose or permanently discontinue based on severity of adverse reaction [see Dosage and Administration (2.5 )] . Provide standard anti-diarrheal agents, administer intravenous fluids to prevent dehydration and replace electrolytes as clinically indicated.
Anorexia/Weight Loss
In patients with multiple myeloma who received XPOVIO 100 mg once weekly (BOSTON, n=195), anorexia was reported in 35% of patients and Grade 3 anorexia was reported in 3.6% of patients. The median time to onset of the first event was 35 days. Permanent discontinuations due to anorexia occurred in 2.1% of patients. Weight loss was reported in 26% of patients and Grade 3 weight loss was reported in 2.1% of patients. The median time to onset of the first event was 58 days. Permanent discontinuation due to weight loss occurred in 1% of patients.
In patients with multiple myeloma who received XPOVIO 80 mg twice weekly (STORM n=202), anorexia was reported as an adverse reaction in 53% of patients and Grade 3 anorexia occurred in 5% of patients. The median time to onset of anorexia was 8 days. Weight loss was reported as an adverse reaction in 47% of patients and Grade 3 weight loss occurred in 1% of patients treated with XPOVIO. The median time to onset of weight loss was 15 days.
In patients with DLBCL (SADAL, n=134), anorexia was reported as an adverse reaction in 37% of patients and Grade 3 anorexia occurred in 3.7% of patients treated with XPOVIO. Weight loss (Grade 1-2) was reported as an adverse reaction in 30% of patients.
Monitor weight, nutritional status, and volume status at baseline and throughout treatment. Monitor more frequently during the first three months of treatment. Interrupt, reduce dose or permanently discontinue based on severity of adverse reaction [see Dosage and Administration (2.5 )]. Provide nutritional support, fluids, and electrolyte repletion as clinically indicated.
Hyponatremia
XPOVIO can cause severe or life-threatening hyponatremia [see Adverse Reactions (6.1 )] .
In patients with multiple myeloma who received XPOVIO 100 mg once weekly (BOSTON, n=195), hyponatremia was reported in 58% of patients and Grade 3-4 hyponatremia was reported in 14% of patients. The median time to first onset was 21 days for any grade hyponatremia and the median time to first onset for Grade 3 or 4 hyponatremia was 22 days.
In patients with multiple myeloma who received XPOVIO 80 mg twice weekly (STORM, n=202), hyponatremia was reported as an adverse reaction in 39% of patients and Grade 3 or 4 hyponatremia was reported in 22% of patients. The median time to onset of the first event was 8 days.
In patients with DLBCL (SADAL, n=134), hyponatremia developed in 62% of patients and Grade 3 hyponatremia developed in 16% of patients treated with XPOVIO. In approximately 63% of cases, hyponatremia occurred in the context of gastrointestinal toxicity such as nausea, vomiting, diarrhea, dehydration, and anorexia.
Monitor sodium level at baseline and throughout treatment. Monitor more frequently during the first two months of treatment. Correct sodium levels for concurrent hyperglycemia (serum glucose >150 mg/dL) and high serum paraprotein levels. Assess hydration status and manage hyponatremia per clinical guidelines, including intravenous saline and/or salt tablets as appropriate and dietary review. Interrupt, reduce dose or permanently discontinue based on severity of the adverse reaction [see Dosage and Administration (2.5 )].
Serious Infection
XPOVIO can cause serious and fatal infections. Most of these infections were not associated with Grade 3 or higher neutropenia [see Adverse Reactions (6.1 )].
In patients with multiple myeloma who received XPOVIO 100 mg once weekly (BOSTON, n=195), 69% of patients experienced any grade of infection. Grade ≥3 infections were reported in 32% of patients, and deaths from infections occurred in 3.1% of patients. The most frequently reported Grade ≥3 infection was pneumonia in 14% of patients, followed by sepsis in 4.1% and upper respiratory tract infection in 3.6% of patients.
In patients with multiple myeloma who received XPOVIO 80 mg twice weekly (STORM, n=202), 52% of patients experienced any grade of infection. Grade ≥3 infections were reported in 25% of patients, and deaths from infections occurred in 4% of patients within 30 days of last treatment. Upper respiratory tract infection of any grade occurred in 21%, pneumonia in 13%, and sepsis in 6% of patients. The most frequently reported Grade ≥3 infections were pneumonia in 9% of patients, followed by sepsis in 6%. The median time to onset was 54 days for pneumonia and 42 days for sepsis.
In patients with DLBCL (SADAL, n=134), 25% of patients experienced Grade 3 or higher infection and 21% had an infection-related serious adverse reaction; 49% developed an infection of any grade, most frequently involving the upper or lower respiratory tract. The most frequently reported Grade ≥3 infections were lower respiratory tract infections in 9% of patients (including pneumonia in 6%), followed by sepsis (6%). The median time to onset of Grade ≥3 infection was 42 days.
Atypical infections reported after XPOVIO include, but are not limited to, fungal pneumonia and herpes virus infection.
Monitor for signs and symptoms of infection, evaluate and treat promptly.
Neurological Toxicity
XPOVIO can cause life-threatening neurological toxicities [see Adverse Reactions (6.1 )] .
In patients with multiple myeloma who received XPOVIO 100 mg once weekly (BOSTON, n=195), neurological adverse reactions (excluding peripheral neuropathy) including dizziness, syncope, depressed level of consciousness, vertigo, amnesia, and mental status changes (including delirium and confusional state) occurred in 26% of patients and severe events (Grade 3-4) occurred in 3.6% of patients. The median time to the first event was 29 days. Permanent discontinuation due to neurological adverse reactions occurred in 2.1% of patients.
In patients with multiple myeloma who received XPOVIO 80 mg twice weekly (STORM, n=202), neurological adverse reactions, including dizziness, syncope, depressed level of consciousness, and mental status changes (including delirium and confusional state) occurred in 30% of patients and severe events (Grade 3-4) occurred in 9% of patients. The median time to the first event was 15 days.
In patients with DLBCL (SADAL, n=134), neurological adverse reactions occurred in 25% of patients and severe events (Grade 3-4) occurred in 6% of patients treated with XPOVIO. The most frequent manifestations were dizziness (16%) and mental status changes (11%), including confusion, cognitive disorders, somnolence, hallucination, delirium, and depressed level of consciousness. Syncope occurred in 2.2% of patients. The median time to the first event was 28 days. Among patients with such neurological adverse reactions, 68% recovered with a median time to recovery of 14 days.
Coadministration of XPOVIO with other products that cause dizziness or mental status changes may increase the risk of neurological toxicity.
Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, until the neurological toxicity fully resolves. Optimize hydration status, hemoglobin level, and concomitant medications to avoid exacerbating dizziness or mental status changes. Institute fall precautions as appropriate.
Embryo-Fetal Toxicity
Based on data from animal studies and its mechanism of action, XPOVIO can cause fetal harm when administered to a pregnant woman. Selinexor administration to pregnant animals during organogenesis resulted in structural abnormalities and alterations to growth at exposures below those occurring clinically at the recommended dose.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with a female partner of reproductive potential to use effective contraception during treatment with XPOVIO and for 1 week after the last dose [see Use in Specific Populations (8.1 , 8.3 )] .
Cataract
New onset or exacerbation of cataract has occurred during treatment with XPOVIO [see Adverse Reactions (6.1 )]. In patients with multiple myeloma who received XPOVIO 100 mg once weekly (BOSTON, n=195), the incidence of new onset or worsening cataracts requiring clinical intervention was reported in 22% of patients. The median time to new onset of cataract was 228 days and was 237 days for worsening of cataract in patients presenting with cataract at start of XPOVIO therapy. Treatment of cataracts usually requires surgical removal of the cataract.
ADVERSE REACTIONS
The following clinically significant adverse reactions are described in detail in other labeling sections:
- Thrombocytopenia [see Warnings and Precautions (5.1 )] .
- Neutropenia [see Warnings and Precautions (5.2 )].
- Gastrointestinal Toxicity [see Warnings and Precautions (5.3 )].
- Hyponatremia [see Warnings and Precautions (5.4 )].
- Serious Infection [see Warnings and Precautions (5.5 )].
- Neurological Toxicity [see Warnings and Precautions (5.6 )] .
- Cataract [see Warnings and Precautions (5.8 )] .
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Multiple Myeloma
XPOVIO in Combination with Bortezomib and Dexamethasone (XVd)
The safety of XPOVIO in combination with bortezomib and dexamethasone was evaluated in BOSTON [see Clinical Studies (14.1 )]. Patients were randomized to receive XPOVIO 100 mg orally once weekly in combination with bortezomib and dexamethasone (XVd) (n=195) or bortezomib and dexamethasone (Vd) (n=204). Among patients who received XPOVIO, the median duration of XPOVIO treatment was 29 weeks (range: 1 to 120 weeks) and the median dose was 80 mg (range: 30 to 137 mg) per week.
Serious adverse reactions occurred in 52% of patients who received XPOVIO in combination with bortezomib and dexamethasone. Serious adverse reactions in >3% of patients included pneumonia (14%), sepsis, diarrhea and vomiting (4% each). Fatal adverse reactions occurred in 6% of patients within 30 days of last treatment, including pneumonia (n=3) and sepsis (n=3).
Grade ≥2 peripheral neuropathy, a pre-specified key secondary endpoint, was lower in the XVd arm (21%) compared to the Vd arm (34%); odds ratio 0.50 [95% CI: 0.32, 0.79]. The median treatment duration was 30 weeks (range: 1-120 weeks) in patients who received once weekly XVd as compared to 32 weeks (range: 1-122 weeks) in patients who received twice weekly Vd.
Permanent discontinuation of XPOVIO due to an adverse reaction occurred in 19% of patients. Adverse reactions which resulted in permanent discontinuation of XPOVIO in >2% of patients included fatigue (3.6%), nausea (3.1%), thrombocytopenia, decreased appetite, peripheral neuropathy, and vomiting (2.1% each).
Dosage interruptions of XPOVIO due to an adverse reaction occurred in 83% of patients. Adverse reactions which required dosage interruption in >5% of patients included thrombocytopenia (33%), fatigue (13%), asthenia (12%), pneumonia (11%), upper respiratory tract infection (10%), decreased appetite (9%), neutropenia (8%), pyrexia (8%), nausea (7%), bronchitis (7%), diarrhea (6%), weight decreased (6%), and anemia (5%).
Dose reductions of XPOVIO due to an adverse reaction occurred in 64% of patients. Adverse reactions which required dose reductions in >5% of patients included thrombocytopenia (31%), decreased appetite (8%), nausea, fatigue, weight decreased (7% each), and asthenia (6%).
The most common adverse reactions (≥20% with a difference between arms of >5% compared to Vd) were fatigue, nausea, decreased appetite, diarrhea, peripheral neuropathy, upper respiratory tract infection, weight decreased, cataract, and vomiting. Grade 3-4 laboratory abnormalities (≥10%) were thrombocytopenia, lymphopenia, hypophosphatemia, anemia, hyponatremia, and neutropenia.
Table 7 summarizes the adverse reactions in BOSTON.
Key: X=XPOVIO, Vd=bortezomib-dexamethasone | ||||
a. Fatigue includes fatigue and asthenia. | ||||
b. Peripheral neuropathy includes neuropathy peripheral, peripheral sensory neuropathy, polyneuropathy, peripheral sensorimotor neuropathy, toxic neuropathy, and peripheral motor neuropathy. | ||||
c. Upper respiratory tract infection includes upper respiratory infection, nasopharyngitis, pharyngitis, respiratory syncytial virus infection, respiratory tract infection, rhinitis, and viral upper respiratory tract infection. | ||||
d. Vision blurred includes blurred vision, visual acuity reduced, and visual impairment. | ||||
| Adverse Reaction | Weekly XVd (n=195) | Twice Weekly Vd (n=204) | ||
| All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) | |
| Gastrointestinal Nausea | 50 | 8 | 10 | 0 |
| Diarrhea | 32 | 6 | 25 | <1 |
| Vomiting | 21 | 4.1 | 4.4 | 0 |
| General Conditions Fatigue a | 59 | 21 | 28 | 5 |
| Pyrexia | 15 | 1.5 | 11 | 1 |
| Metabolism and Nutrition Decreased appetite | 35 | 3.6 | 5 | 0 |
| Weight decreased | 26 | 2.1 | 12 | 1 |
| Nervous System Peripheral neuropathy b | 32 | 4.6 | 47 | 9 |
| Dizziness | 12 | <1 | 3.9 | 0 |
| Infections Upper respiratory tract infection c | 29 | 3.6 | 22 | 1.5 |
| Eye Disorders Cataract | 22 | 9 | 6 | 1.5 |
| Vision blurred d | 13 | <1 | 6 | 0 |
Clinically relevant adverse reactions in <10% of patients who received XPOVIO in combination with bortezomib and dexamethasone included:
- Neurologic disorders: mental status changes (9%) and syncope (3.6%)
Table 8 summarizes selected laboratory abnormalities in BOSTON.
The denominator used to calculate the rate varied from 91 to 201 based on the number of patients with at least one post-treatment value. | ||||
a. Includes one fatal anemia. | ||||
| Laboratory Abnormality | Weekly XVd | Twice Weekly Vd | ||
| All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) | |
| Hematologic Platelet count decrease | 92 | 43 | 51 | 19 |
| Lymphocyte count decrease | 77 | 38 | 70 | 27 |
| Hemoglobin decrease | 71 | 17 | 51 a | 12 |
| Neutrophil count decrease | 48 | 12 | 19 | 7 |
| Chemistry Glucose increase | 62 | 3.8 | 47 | 4.1 |
| Phosphate decrease | 61 | 23 | 42 | 11 |
| Sodium decrease | 58 | 14 | 25 | 3 |
| Calcium decrease | 55 | 2.1 | 47 | 1 |
| Blood urea nitrogen increase | 41 | 5 | 40 | 5 |
| Creatinine increase | 28 | 3.6 | 24 | 1.5 |
| Potassium decrease | 27 | 6 | 22 | 3.5 |
| Magnesium decrease | 27 | <1 | 23 | 1.5 |
| Potassium increase | 18 | 4.1 | 21 | 2.5 |
| Hepatic ALT increase | 33 | 3.1 | 30 | <1 |
| Albumin decrease | 27 | <1 | 35 | <1 |
| AST increase | 24 | 1.5 | 19 | <1 |
| Bilirubin increase | 16 | 1 | 13 | 2 |
| ALP increase | 12 | 0 | 16 | <1 |
XPOVIO in Combination with Dexamethasone (Xd)
The safety of XPOVIO in combination with dexamethasone was evaluated in STORM [see Clinical Studies (14.1 )]. Patients received XPOVIO 80 mg orally with dexamethasone 20 mg on Days 1 and 3 of every week (n=202). The median duration of XPOVIO treatment was 8 weeks (range: 1 to 60 weeks). The median dose was 115 mg (range: 36 to 200 mg) per week.
Fatal adverse reactions occurred in 9% of XPOVIO treated patients. Serious adverse reactions occurred in 58% of patients.
The treatment discontinuation rate due to adverse reactions was 27%; 53% of patients had a reduction in the XPOVIO dose, and 65% had the dose of XPOVIO interrupted. Thrombocytopenia was the leading cause of dose modification, resulting in dose reduction and/or interruption in >25% of patients. The most frequent adverse reactions requiring permanent discontinuation in 4% or greater of patients who received XPOVIO included fatigue, nausea, and thrombocytopenia.
Table 9 summarizes the adverse reactions in STORM.
a. Thrombocytopenia includes thrombocytopenia and platelet count decreased. | ||
b. Fatigue includes fatigue and asthenia. | ||
c. Anemia includes anemia and hematocrit decreased. | ||
d. Neutropenia includes neutropenia and neutrophil count decreased. | ||
e. Dyspnea includes dyspnea, dyspnea exertional, and dyspnea at rest. | ||
f. Upper respiratory tract infection includes upper respiratory tract infection, respiratory tract infection, pharyngitis, nasopharyngitis, bronchitis, bronchiolitis, respiratory syncytial virus infection, parainfluenza virus infection, rhinitis, rhinovirus infection, and adenovirus infection. | ||
g. Cough includes cough, productive cough, and upper-airway cough syndrome. | ||
h. Mental status changes includes mental status changes, confusional state, and delirium. | ||
i. Hypercreatininemia includes hypercreatininemia and hypercreatinemia. | ||
j. Pneumonia includes pneumonia, atypical pneumonia, lung infection, lower respiratory tract infection, pneumocystis jirovecii pneumonia, pneumonia aspiration, pneumonia influenzal, and pneumonia viral. | ||
k. Includes fatal event. | ||
| Adverse Reaction | XPOVIO 80 mg twice weekly + Dexamethasone (n=202) | |
| All Grades (%) | Grades ≥3 (%) | |
| Thrombocytopenia a | 74 | 61 |
| Fatigue b | 73 | 22 |
| Nausea | 72 | 9 |
| Anemia c | 59 | 40 |
| Decreased appetite | 53 | 4.5 |
| Weight decreased | 47 | 0.5 |
| Diarrhea | 44 | 6 |
| Vomiting | 41 | 3.5 |
| Hyponatremia | 39 | 22 |
| Neutropenia d | 34 | 21 |
| Leukopenia | 28 | 11 |
| Constipation | 25 | 1.5 |
| Dyspnea e | 24 | 3.5 k |
| Upper respiratory tract infection f | 21 | 3 |
| Cough g | 16 | 0 |
| Mental status changes h | 16 | 7 |
| Pyrexia | 16 | 0.5 |
| Hyperglycemia | 15 | 7 |
| Dizziness | 15 | 0 |
| Insomnia | 15 | 2 |
| Lymphopenia | 15 | 10 |
| Dehydration | 14 | 3.5 |
| Hypercreatininemia i | 14 | 2 |
| Pneumonia j | 13 | 9 k |
| Epistaxis | 12 | 0.5 |
| Hypokalemia | 12 | 3.5 |
| Dysgeusia | 11 | 0 |
| Vision blurred | 10 | 0.5 |
| Headache | 10 | 0 |
Diffuse Large B-Cell Lymphoma
The safety of XPOVIO was evaluated in SADAL [see Clinical Studies (14.2 )]. Patients received XPOVIO 60 mg orally on Days 1 and 3 of every week (n=134). The study required an absolute neutrophil count ≥1000/μL, platelet count ≥75,000/μL, hepatic transaminases ≤2.5 times upper limit of normal (ULN) unless abnormal from lymphoma, and bilirubin ≤2 times ULN. The study permitted a maximum of 5 prior systemic regimens for DLBCL. Antiemetic prophylaxis with a 5HT-3 receptor antagonist was required. The median duration of XPOVIO treatment was 2.1 months (range: 1 week to 3.7 years) with 38% receiving at least 3 months and 22% receiving at least 6 months of treatment. The median exposure was 100 mg per week.
Fatal adverse reactions occurred in 3.7% of patients within 30 days and 5% of patients within 60 days of last treatment; the most frequent fatal adverse reaction was infection (4.5% of patients). Serious adverse reactions occurred in 46% of patients who received XPOVIO; the most frequent serious adverse reaction was infection (21% of patients).
Discontinuation due to adverse reactions occurred in 17% of patients who received XPOVIO. Adverse reactions which results in discontinuation in ≥2% of patients included: infection, fatigue, thrombocytopenia, and nausea.
Adverse reactions led to XPOVIO dose interruption in 61% of patients and dose reduction in 49%, with 17% of all patients having 2 or more dose reductions. The median time to first dose modification (reduction or interruption) was 4 weeks, with the leading causes being thrombocytopenia (40% of all patients), neutropenia (16%), fatigue (16%), nausea (10%), and anemia (10%). The median time to first dose reduction was 6 weeks, with 83% of first dose reductions occurring within the first 3 months.
The most common adverse reactions, excluding laboratory abnormalities, in ≥20% of patients were fatigue, nausea, diarrhea, decreased appetite, weight decreased, constipation, vomiting, and pyrexia. Table 10 summarizes selected adverse reactions in SADAL.
a. Fatigue includes fatigue and asthenia. | ||
b. Edema includes edema, swelling, swelling face, edema peripheral, peripheral swelling, acute pulmonary edema. | ||
c. Diarrhea includes diarrhea, post-procedural diarrhea, gastroenteritis. | ||
d. Abdominal pain includes abdominal pain, abdominal pain upper, abdominal discomfort, epigastric discomfort. | ||
e. Decreased appetite includes decreased appetite and hypophagia. | ||
f. Cough includes cough and productive cough. | ||
g. Dyspnea includes dyspnea and dyspnea exertional. | ||
h. Upper respiratory tract infection includes upper respiratory tract infection, sinusitis, nasopharyngitis, pharyngitis, rhinitis, viral upper respiratory infection. | ||
i. Urinary tract infection includes urinary tract infection and specific types of urinary tract infection. | ||
j. Dizziness includes dizziness and vertigo. | ||
k. Taste disorder includes taste disorder, dysgeusia, ageusia. | ||
l. Mental status changes include confusional state, amnesia, cognitive disorder, hallucination, delirium, somnolence, depressed level of consciousness, memory impairment. | ||
m. Peripheral neuropathy includes peripheral neuropathy, peripheral sensory neuropathy, sensory disturbance, paresthesia, neuralgia. | ||
n. Musculoskeletal pain includes musculoskeletal pain, back pain, musculoskeletal chest pain, neck pain, pain in extremity, bone pain. | ||
o. Hemorrhage includes hemorrhage, hematoma, hematuria, epistaxis, rectal hemorrhage, injection site hematoma, subdural hematoma, upper gastrointestinal hemorrhage, corneal bleeding. | ||
p. Vision blurred includes vision blurred, visual acuity reduced, visual impairment. | ||
| Adverse Reaction | XPOVIO 60 mg twice weekly (n=134) | |
| All Grades (%) | Grade 3 or 4 (%) | |
| General Conditions Fatigue a | 63 | 15 |
| Pyrexia | 22 | 4.5 |
| Edema b | 17 | 2.2 |
| Gastrointestinal Nausea | 57 | 6 |
| Diarrhea c | 37 | 3.0 |
| Constipation | 29 | 0 |
| Vomiting | 28 | 1.5 |
| Abdominal pain d | 10 | 0 |
| Adverse Reaction | XPOVIO 60 mg twice weekly (n=134) | |
| All Grades (%) | Grade 3 or 4 (%) | |
| Metabolism and Nutrition Decreased appetite e | 37 | 3.7 |
| Weight decreased | 30 | 0 |
| Respiratory Cough f | 18 | 0 |
| Dyspnea g | 10 | 1.5 |
| Infections Upper respiratory tract infection h | 17 | 1.5 |
| Pneumonia | 10 | 6 |
| Urinary tract infection i | 10 | 3 |
| Nervous System Dizziness j | 16 | 0.7 |
| Taste disorder k | 13 | 0 |
| Mental status changes l | 11 | 3.7 |
| Peripheral neuropathy, sensory m | 10 | 0 |
| Musculoskeletal Musculoskeletal pain n | 15 | 2.2 |
| Vascular Hypotension | 13 | 3.0 |
| Hemorrhage o | 10 | 0.7 |
| Eye Disorders Vision blurred p | 11 | 0.7 |
Clinically relevant adverse reactions in <10% of patients who received XPOVIO included:
- Injury: fall (8%)
- Metabolic and nutrition disorders: dehydration (7%)
- Neurologic disorders: headache (4.5%), syncope (2.2%)
- Infection: sepsis (6%), herpes virus infection (3%)
- Eye disorders: cataract (3.7%)
- Blood and lymphatic disorders: febrile neutropenia (3%)
- Cardiac disorders: cardiac failure (3%)
Table 11 summarizes selected new or worsening laboratory abnormalities in SADAL. Grade 3-4 laboratory abnormalities in ≥15% included thrombocytopenia, lymphopenia, neutropenia, anemia, and hyponatremia. Grade 4 laboratory abnormalities in ≥5% were thrombocytopenia (18%), lymphopenia (5%), and neutropenia (9%).
The denominator used to calculate the rate varied from 107 to 128 based on the number of patients with at least one post-treatment value. | ||
a. Not fasting. | ||
b. CK increase was not associated with reports of myopathy or myalgia. | ||
| Laboratory Abnormality | XPOVIO 60 mg twice weekly | |
| All Grades (%) | Grade 3 or 4 (%) | |
| Hematologic Platelet count decrease | 86 | 49 |
| Hemoglobin decrease | 82 | 25 |
| Lymphocyte count decrease | 63 | 37 |
| Neutrophil count decrease | 58 | 31 |
| Chemistry Sodium decrease | 62 | 16 |
| Glucose increase | 57 a | 5 |
| Creatinine increase | 47 | 3.9 |
| Phosphate decrease | 34 | 11 |
| Magnesium decrease | 30 | 2.6 |
| Calcium decrease | 30 | 0.9 |
| Potassium increase | 26 | 3.9 |
| Potassium decrease | 23 | 7 |
| CK increase b | 21 | 1.9 |
| Hepatic ALT increase | 29 | 0.8 |
| Albumin decrease | 25 | 0 |
| AST increase | 24 | 3.1 |
| Bilirubin increase | 16 | 1.6 |
DESCRIPTION
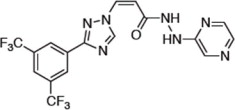
Selinexor is a nuclear export inhibitor. Selinexor is (2 Z )-3-{3-[3,5-bis(trifluoromethyl)phenyl]-1 H -1,2,4-triazol-1yl}- N '-(pyrazin-2-yl)prop-2-enehydrazide. It is a white to off-white powder and has the molecular formula C 17 H 11 F 6 N 7 O and a molecular mass of 443.31 g/mol. The molecular structure is shown below:
XPOVIO tablets for oral dosing are supplied in six strengths, with each tablet containing 10 mg, 20 mg, 40 mg, 50 mg, 60 mg, or 80 mg of selinexor as the active ingredient. The inactive ingredients are colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, Opadry 200 clear, Opadry II blue, povidone K30, and sodium lauryl sulfate.
CLINICAL PHARMACOLOGY
Mechanism of Action
In nonclinical studies, selinexor reversibly inhibits nuclear export of tumor suppressor proteins (TSPs), growth regulators, and mRNAs of oncogenic proteins by blocking exportin 1 (XPO1). XPO1 inhibition by selinexor leads to accumulation of TSPs in the nucleus and reductions in several oncoproteins, such as c-myc and cyclin D1, cell cycle arrest, and apoptosis of cancer cells. Selinexor demonstrated pro-apoptotic activity in vitro in multiple myeloma cells and showed anti-tumor activity in murine xenograft models of multiple myeloma and diffuse large B cell lymphoma. The combination of selinexor and dexamethasone or bortezomib demonstrated synergistic cytotoxic effects in multiple myeloma in vitro and increased anti-tumor activity in murine xenograft multiple myeloma models in vivo, including those resistant to proteasome inhibitors.
Pharmacodynamics
An increase in selinexor exposure was associated with an increase in the probability of dose modification and some adverse reactions.
Cardiac Electrophysiology
The effect of multiple doses of XPOVIO, up to 175 mg per dose (1.75 times the maximum approved recommended dose), on the QTc interval was evaluated in patients with heavily pretreated hematologic malignancies. XPOVIO had no large effect (i.e. no greater than 20 ms) on QTc interval at the therapeutic dose level.
Pharmacokinetics
Selinexor C max and AUC increased proportionally over a dose range from 3 mg/m 2 to 85 mg/m 2 (0.05 to 1.44) times the maximum approved recommended dose, based on 1.7 m 2 body surface area. No clinically relevant accumulation at steady state was observed. Selinexor C max and AUC 0-INF after administration of a single dose of XPOVIO in patients with hematologic malignancies are presented in Table 12 .
| Mean (SD) | XPOVIO Dose | ||
| 60 mg | 80 mg | 100 mg | |
| C max (ng/mL) | 442 (188) | 680 (124) | 693 (201) |
| AUC 0-INF (ng·h/mL) | 4,096 (1,185) | 5,386 (1,116) | 6,998 (818) |
Absorption
The C max is reached within 4 hours following oral administration of XPOVIO.
Effect of Food
Concomitant administration of a high-fat meal (800 to 1,000 calories with approximately 50% of total caloric content of the meal from fat) did not affect the pharmacokinetics of selinexor to a clinically significant extent.
Distribution
The apparent volume of distribution of selinexor is 133 L in patients with cancer. The protein binding of selinexor is 95%.
Elimination
Following a single dose of XPOVIO, the mean half-life is 6 to 8 hours. The apparent total clearance of selinexor is 18.6 L/h in patients with cancer.
Metabolism
Selinexor is metabolized by CYP3A4, multiple UDP-glucuronosyltransferases (UGTs) and glutathione Stransferases (GSTs).
Specific Populations
No clinically significant differences in the pharmacokinetics of selinexor were observed based on age (18 to 94 years old), sex, body weight (36 to 168 kg), ethnicity, mild to severe renal impairment (CL CR : 15 to 89 mL/min, estimated by the Cockcroft-Gault equation), and disease type (hematological non-DLBCL, solid tumor, DLBCL). The effect of end-stage renal disease (CL CR <15 mL/min) or hemodialysis on selinexor pharmacokinetics is unknown.
Patients with Hepatic Impairment
In patients with mild (total bilirubin ≤1x ULN and AST >1x ULN, or total bilirubin >1 to 1.5x ULN and any AST) or moderate (total bilirubin >1.5 to 3x ULN and any AST, n=7) hepatic impairment the pharmacokinetics of selinexor was comparable to patients with normal hepatic function (total bilirubin and AST
In patients with severe hepatic impairment (total bilirubin >3 ULN and any AST, n=6) there was approximately 32% increase in total and free selinexor exposure compared to patients with normal hepatic function [see Dosage and Administration (2.6 ) and Use in Specific Populations (8.6 )] .
Drug Interaction Studies
Clinical Studies
Acetaminophen: No clinically significant differences in selinexor pharmacokinetics were observed when co-administered with acetaminophen (up to 1,000 mg daily dose of acetaminophen).
Clarithromycin (a strong CYP3A4 inhibitor): No clinically significant differences in selinexor pharmacokinetics were observed when co-administered with clarithromycin (up to 1,000 mg daily dose of clarithromycin).
Carbamazepine (a strong CYP3A4 inducer): No clinically significant differences in selinexor pharmacokinetics were observed when co-administered with carbamazepine (up to 300 mg twice-daily dose of carbamazepine).
In vitro Studies
CYP Enzymes: Selinexor does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP3A4/5. Selinexor is not a CYP3A4, CYP1A2, or CYP2B6 inducer.
Non-CYP Enzyme Systems : Selinexor is a substrate of UGTs and GSTs.
Transporter Systems: Selinexor inhibits OATP1B3 but does not inhibit other solute carrier (SLC) transporters.
Selinexor is not a substrate of P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2, MATE1, or MATE2-K.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with selinexor.
Selinexor was not mutagenic in vitro in a bacterial reverse mutation (Ames) assay and was not clastogenic in either the in vitro cytogenetic assay in human lymphocytes or in the in vivo rat micronucleus assay.
Fertility studies in animals have not been conducted with selinexor. In repeat-dose oral toxicity studies, selinexor was administered for up to 13 weeks in rats and monkeys. Reduced sperm, spermatids, and germ cells in epididymides and testes were observed in rats at ≥1 mg/kg, decreased ovarian follicles were observed in rats at ≥2 mg/kg, and single cell necrosis of testes was observed in monkeys at ≥1.5 mg/kg. These dose levels resulted in systemic exposures approximately 0.11, 0.28, and 0.53 times, respectively, the exposure (AUC last ) in humans at the recommended human dose of 80 mg.
CLINICAL STUDIES
Relapsed or Refractory Multiple Myeloma
XPOVIO Combination with Bortezomib and Dexamethasone (XVd)
The efficacy of XPOVIO in combination with bortezomib and dexamethasone was evaluated in BOSTON (NCT03110562). BOSTON was a global, randomized, open label, active-controlled trial in adult patients who had received 1 to 3 prior anti-MM regimens. Prior treatment with bortezomib or other PI was allowed.
Patients with Grade 2 or higher peripheral neuropathy at study entry were excluded.
Patients were randomized to receive one of the following:
- XPOVIO 100 mg orally once weekly on Days 1, 8, 15, 22, 29 in combination with bortezomib 1.3 mg/m 2 administered subcutaneously once weekly on Days 1, 8, 15, 22 and dexamethasone 20 mg taken orally twice weekly on Days 1, 2, 8, 9, 15, 16, 22, 23, 29, and 30 of each 35-day cycle [XVd arm] or
- Bortezomib 1.3 mg/m 2 administered subcutaneously twice weekly on Days 1, 4, 8, 11 and dexamethasone 20 mg taken orally four times weekly on Days 1, 2, 4, 5, 8, 9, 11, 12 of each 21-day cycle for the first 8 cycles, followed by bortezomib 1.3 mg/m 2 administered subcutaneously once weekly on Days 1, 8, 15, 22 and dexamethasone 20 mg taken orally twice weekly on Days 1, 2, 8, 9, 15, 16, 22, 23, 29, and 30 of each 35-day cycle (Cycle ≥9) [Vd arm].
Treatment continued in both arms until disease progression or unacceptable toxicity. Randomization was stratified based on prior proteasome inhibitor therapies exposure (yes versus no), number of prior regimens (1 versus >1), Stage (III versus I or II) according to the Revised-International Staging System (RISS) and region. Upon confirmed progressive disease (PD), patients in the Vd arm could receive XPOVIO in combination with bortezomib and dexamethasone (XVd) or XPOVIO 100 mg taken orally on Days 1, 8, 15, 22, 29 with dexamethasone 20 mg taken orally on Days 1, 2, 8, 9, 15, 16, 22, 23, 29, and 30 of each 35-day cycle.
A total of 402 patients were randomized: 195 to XVd arm and 207 to Vd arm. Baseline patient demographics and disease characteristics are summarized in Table 13 and Table 14 , respectively.
| Characteristic | XVd (n=195) | Vd (n=207) |
| Median age, years (range) | 66 (40, 87) | 67 (38, 90) |
| Age distribution, n (%) <65 years | 86 (44) | 75 (36) |
| 65 – 74 years | 75 (38) | 85 (41) |
| ≥75 years | 34 (17) | 47 (23) |
| Sex, n (%) Male | 115 (59) | 115 (56) |
| Female | 80 (41) | 92 (44) |
| Race, n (%) White | 161 (83) | 165 (80) |
| Black or African American | 4 (2) | 7 (3) |
| Asian | 25 (13) | 25 (12) |
| Other | 0 | 1 (0.5) |
| Missing | 5 (3) | 9 (4) |
a. Includes any of del(17p)/p53, t(14;16), t(4;14), 1q21. | ||
| Parameter | XVd (n=195) | Vd (n=207) |
| Median years from diagnosis to randomization (range) | 3.81 (0.4, 23.0) | 3.59 (0.4, 22.0) |
| ECOG performance status score, n (%) 0-1 | 175 (90) | 191 (92) |
| ≥2 | 20 (10) | 16 (8) |
| Creatinine Clearance, n (%), mL per minute <30 | 3 (1.5) | 10 (5) |
| 30 to 59 | 53 (27) | 60 (29) |
| ≥60 | 139 (71) | 137 (66) |
| Revised International Staging System at Baseline, n (%) I | 56 (29) | 52 (25) |
| II | 117 (60) | 125 (60) |
| III | 12 (6) | 16 (8) |
| Unknown | 10 (5) | 14 (7) |
| Number of Prior Therapies, n (%) 1 2 3 | 99 (51) 65 (33) 31 (16) | 99 (48) 64 (31) 44 (21) |
| Type of known prior therapy, n (%) Stem Cell transplantation | 76 (39) | 63 (30) |
| Lenalidomide | 77 (39) | 77 (37) |
| Pomalidomide | 11 (6) | 7 (3) |
| Bortezomib | 134 (69) | 145 (70) |
| Carfilzomib | 20 (10) | 21 (10) |
| Daratumumab | 11 (6) | 6 (3) |
| Median weeks since end of last prior therapy, (range) | 48 (1, 1088) | 42 (2, 405) |
| Known high-risk cytogenetics a , n (%) | 97 (50) | 95 (46) |
Efficacy was based on progression free survival (PFS) according to the International Myeloma Working Group (IMWG) Uniform Response Criteria for Multiple Myeloma, as assessed by an Independent Review Committee (IRC). Efficacy results based on a preplanned PFS interim analysis, are shown in Table 15 and Figure 1 .
a. Hazard ratio is based on stratified Cox's proportional hazard regression modeling, p-value based on stratified log-rank test. | ||
Median follow up of 15.1 months at the time of the analysis. | ||
b. The pre-planned PFS interim analysis boundary of statistical significance was defined as a p-value <0.0103. | ||
c. Includes sCR + CR + VGPR + PR, p value based on Cochran-Mantel-Haenszel test. | ||
d. Includes sCR + CR + VGPR, p value based on Cochran-Mantel-Haenszel test. | ||
| XVd (n=195) | Vd (n=207) | |
| Progression Free Survival (PFS) a Hazard Ratio [95% CI] | 0.70 [0.53, 0.93] | |
| One-sided p-value b | 0.0075 | |
| Median PFS in months [95% CI] | 13.9 (11.7, Not Reached) | 9.5 (7.6, 10.8) |
| Overall Response Rate (ORR) c , n (%) | 149 (76.4) | 129 (62.3) |
| 95% CI | (69.8, 82.2) | (55.3, 68.9) |
| One-sided p-value | 0.0012 | |
| Stringent Complete Response (sCR) | 19 (10) | 13 (6) |
| Complete Response (CR) | 14 (7) | 9 (4) |
| Very Good Partial Response (VGPR) | 54 (28) | 45 (22) |
| Partial Response (PR) | 62 (32) | 62 (30) |
| ≥ VGPR Response Rate d , n (%) | 87 (44.6) | 67 (32.4) |
| 95% CI | (37.5, 51.9) | (26.0, 39.2) |
| One-sided p-value | 0.0082 | |
Figure 1: Kaplan-Meier Curve of PFS (BOSTON)
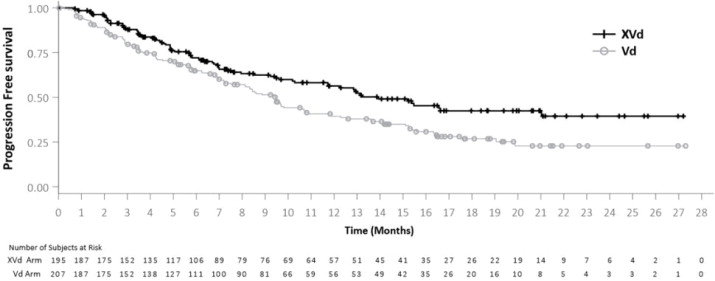
The median time to response was 1.4 months in the XVd arm and 1.6 months in the Vd arm. The median duration of response, among responding patients, was 20.3 months and 12.9 months in the XVd and Vd arms, respectively.
XPOVIO Combination with Dexamethasone (Xd)
The efficacy of XPOVIO plus dexamethasone was evaluated in STORM (KCP-330-012; NCT02336815). STORM was a multicenter, single-arm, open-label study of adults with relapsed or refractory multiple myeloma (RRMM). STORM Part 2 included 122 patients with RRMM who had previously received three or more antimyeloma treatment regimens including an alkylating agent, glucocorticoids, bortezomib, carfilzomib, lenalidomide, pomalidomide, and an anti-CD38 monoclonal antibody; and whose myeloma was documented to be refractory to glucocorticoids, a proteasome inhibitor, an immunomodulatory agent, an anti-CD38 monoclonal antibody, and to the last line of therapy.
In STORM Part 2, a total of 122 patients received XPOVIO 80 mg orally in combination with dexamethasone 20 mg orally on Days 1 and 3 of every week. Treatment continued until disease progression or unacceptable toxicity. Eighty-three patients had RRMM that was refractory to bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab. Baseline patient demographics and disease characteristics of these 83 patients are summarized in Table 16 and Table 17 , respectively.
Efficacy was based on overall response rate (ORR), as assessed by an Independent Review Committee (IRC) based on the International Myeloma Working Group (IMWG) Uniform Response Criteria for Multiple
Myeloma. The approval of XPOVIO was based upon the efficacy and safety in a prespecified subgroup analysis of the 83 patients whose disease was refractory to bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab, as the benefit-risk ratio appeared to be greater in this more heavily pretreated population than in the overall trial population. Overall response rate results are presented in Table 18 . The median time to first response was 4 weeks (range: 1 to 10 weeks). The median duration of response was 3.8 months (95% CI: 2.3, not estimable).
| Demographic | STORM (n=83) |
| Median age, years (range) | 65 (40, 86) |
| Age category, n (%) | |
| <65 years | 40 (48) |
| 65 – 74 years | 31 (37) |
| ≥75 years | 12 (15) |
| Sex, n (%) | |
| Male | 51 (61) |
| Female | 32 (39) |
| Race, n (%) | |
| White | 58 (70) |
| Black or African American | 13 (16) |
| Asian | 2 (2) |
| Native Hawaiian or other Pacific Islander | 1 (1) |
| Other | 6 (7) |
| Missing | 3 (4) |
a. Includes any of del(17p)/p53, t(14; 16), t(4; 14), 1q21. | |
| Parameter | STORM (n=83) |
| Median years from diagnosis to start of study treatment (range) | 7 (1, 23) |
| Prior treatment regimens, median (range) | 8 (4, 18) |
| Documented refractory status, n (%) Lenalidomide | 83 (100) |
| Pomalidomide | 83 (100) |
| Bortezomib | 83 (100) |
| Carfilzomib | 83 (100) |
| Daratumumab | 83 (100) |
| Documented refractory status to specific combinations, n (%) Bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab | 83 (100) |
| Daratumumab in any combination | 57 (69) |
| Daratumumab as single agent (+/- dexamethasone) | 26 (31) |
| Previous stem cell transplant, n (%) | 67 (81) |
| Revised International Staging System at Baseline, n (%) I | 10 (12) |
| II | 56 (68) |
| III | 17 (21) |
| Unknown | 0 |
| High-risk cytogenetics a , n (%) | 47 (57) |
a. Includes sCR + CR + VGPR + PR. | |
| Response | STORM (n=83) |
| Overall Response Rate (ORR) a , n (%) | 21 (25.3) |
| 95% CI | 16.4, 36 |
| Stringent Complete Response (sCR) | 1 (1) |
| Complete Response (CR) | 0 |
| Very Good Partial Response (VGPR) | 4 (5) |
| Partial Response (PR) | 16 (19) |
Relapsed or Refractory Diffuse Large B-Cell Lymphoma
The efficacy of XPOVIO monotherapy was evaluated in SADAL (KCP-330-009; NCT02227251). SADAL was a multicenter, single-arm, open-label study of adults with relapsed or refractory DLBCL, not otherwise specified (NOS), after 2 to 5 systemic regimens. Eligible patients were not candidates for autologous hematopoietic stem cell transplantation (HSCT). The study required a minimum of 60 days since last systemic therapy, with a minimum of 98 days in patients with refractory disease (defined as less than partial response) to last systemic therapy.
Patients received XPOVIO 60 mg orally on Days 1 and 3 of each week. Treatment continued until disease progression or unacceptable toxicity. Of 134 patients evaluated, the median age was 67 years (range: 35-91), 59% were male, 79% were White, and 7% were Asian. Most patients (88%) had an ECOG performance status of 0 or 1. The diagnosis was de novo DLBCL not otherwise specified (NOS) in 75% and transformed DLBCL in 23%. The median number of prior systemic therapies was 2 (range: 1-5), with 63% of patients receiving 2 prior systemic therapies, 24% receiving 3 prior therapies, and 10% receiving 4 or 5 prior therapies. Twenty-eight percent had documented refractory disease to the most recent therapy; 30% had prior autologous HSCT. The median time from last systemic therapy to the start of XPOVIO was 5.4 months overall and 3.6 months in the patients with refractory disease.
Efficacy was based on overall response rate (ORR) and duration of response as assessed by an Independent Review Committee (IRC) using Lugano 2014 criteria (Table 19 ). The median time to first response was 8.1 weeks (range: 6.7-16.4 weeks).
| Parameter | XPOVIO 60 mg twice weekly (n=134) |
| ORR per Lugano criteria, n (%) 95% CI, % | 39 (29) 22, 38 |
| Complete Response | 18 (13) |
| Partial Response | 21 (16) |
| Duration of Response Patients maintaining response at 3 months, n/N (%) | 22/39 (56) |
| Patients maintaining response at 6 months, n/N (%) | 15/39 (38) |
| Patients maintaining response at 12 months, n/N (%) | 6/39 (15) |
HOW SUPPLIED/STORAGE AND HANDLING
XPOVIO 10 mg tablets are blue, round, bi-convex, film-coated debossed with “X10” on one side and nothing on the other side.
XPOVIO 20 mg tablets are blue, round, bi-convex, film-coated debossed with “K20” on one side and nothing on the other side.
XPOVIO 40 mg tablets are blue, oval, film-coated, debossed on both sides with “X40”.
XPOVIO 50 mg tablets are blue, oval, film-coated, debossed on both sides with “X50”.
XPOVIO 60 mg tablets are blue, oval, film-coated, debossed on both sides with “X60”.
XPOVIO 80 mg tablets are blue, oval, film-coated, debossed on both sides with “X80”.
Tablets are packaged in a child-resistant blister pack. Four blister packs are supplied per carton. The following nine dose presentations are available:
| Weekly dose | Strength per tablet | Carton (28-day supply) | Blister Pack | NDC |
|---|---|---|---|---|
| 100 mg once weekly | 50 mg | 4 blister packs (8 tablets total in the carton) | Each blister has two 50 mg tablets | Outer carton NDC 72237-103-05 Blister pack NDC 72237-103-15 |
| 80 mg once weekly | 40 mg | 4 blister packs (8 tablets total in the carton) | Each blister has two 40 mg tablets | Outer carton NDC 72237-102-02 Blister pack NDC 72237-102-12 |
| 80 mg once weekly | 80 mg | 4 blister packs (4 tablets total in the carton) | Each blister has one 80 mg tablet | Outer carton NDC 72237-105-01 Blister pack NDC 72237-105-11 |
| 60 mg once weekly | 60 mg | 4 blister packs (4 tablets total in the carton) | Each blister has one 60 mg tablet | Outer carton NDC 72237-104-01 Blister pack NDC 72237-104-11 |
| 40 mg once weekly | 10 mg | 4 blister packs (16 tablets total in the carton) | Each blister has four 10 mg tablets | Outer carton NDC 72237-106-01 Blister pack NDC 72237-106-11 |
| 40 mg once weekly | 40 mg | 4 blister packs (4 tablets total in the carton) | Each blister has one 40 mg tablet | Outer carton NDC 72237-102-07 Blister pack NDC 72237-102-17 |
| 80 mg twice weekly | 20 mg | 4 blister packs (32 tablets total in the carton) | Each blister has eight 20 mg tablets | Outer carton NDC 72237-101-04 Blister pack NDC 72237-101-14 |
| 60 mg twice weekly | 20 mg | 4 blister packs (24 tablets total in the carton) | Each blister has six 20 mg tablets | Outer carton NDC 72237-101-03 Blister pack NDC 72237-101-13 |
| 40 mg twice weekly | 40 mg | 4 blister packs (8 tablets total in the carton) | Each blister has two 40 mg tablets | Outer carton NDC 72237-102-06 Blister pack NDC 72237-102-16 |
Store at or below 30°C (86°F).
Mechanism of Action
In nonclinical studies, selinexor reversibly inhibits nuclear export of tumor suppressor proteins (TSPs), growth regulators, and mRNAs of oncogenic proteins by blocking exportin 1 (XPO1). XPO1 inhibition by selinexor leads to accumulation of TSPs in the nucleus and reductions in several oncoproteins, such as c-myc and cyclin D1, cell cycle arrest, and apoptosis of cancer cells. Selinexor demonstrated pro-apoptotic activity in vitro in multiple myeloma cells and showed anti-tumor activity in murine xenograft models of multiple myeloma and diffuse large B cell lymphoma. The combination of selinexor and dexamethasone or bortezomib demonstrated synergistic cytotoxic effects in multiple myeloma in vitro and increased anti-tumor activity in murine xenograft multiple myeloma models in vivo, including those resistant to proteasome inhibitors.