Get your patient on Yutiq (Fluocinolone Acetonide)
Yutiq prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Yutiq patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
General Dosing Information
For ophthalmic intravitreal injection.
Administration
The intravitreal injection procedure should be carried out under aseptic conditions, which include use of sterile gloves, a sterile drape, a sterile caliper, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection.
The injection procedure for YUTIQ is as follows:
- Just prior to injection, administer topical and/or subconjunctival anesthesia at the injection site (inferotemporal quadrant recommended).
- Administer 2-3 drops of a broad-spectrum microbicide into the lower fornix. The lids may be scrubbed with cotton-tipped applicators soaked with a broad-spectrum microbicide. Place a sterile lid speculum. Have the patient look up and apply additional microbicide solution to the injection site. Allow 30-60 seconds for the topical antiseptic to dry prior to injection of YUTIQ.
- Optimal placement of YUTIQ is inferior to the optic disc and posterior to the equator of the eye. Measure 4 millimeters inferotemporal from the limbus with the aid of callipers for point of entry into the sclera.
- Using sterile procedure, open the sterile foil pouch containing YUTIQ.
- Remove the YUTIQ applicator from the sterile pouch by grasping the barrel of the applicator; do not grasp the plunger.
- Remove the black plunger stop from the plunger.
- Carefully remove the protective cap from the needle and inspect the needle tip to ensure it is not bent.
- Remove the trombone wire from the distal end of the needle. Prior to injection, keep the applicator tip above the horizontal plane to ensure that the YUTIQ implant does not fall out of the applicator.
- Gently displace the conjunctiva so that after withdrawing the needle, the conjunctival and scleral needle entry sites will not align. Care should be taken to avoid contact between the needle and the lid margin or lashes.
- Insert the needle through the conjunctiva and sclera up to the positive stop of the applicator.
- Depress the plunger at the back of the applicator fully to deliver the YUTIQ implant into the back of the eye.
- Remove the YUTIQ applicator from the eye and discard in biohazard sharps container.
- Remove the lid speculum and perform indirect ophthalmoscopy to verify adequate central retinal artery perfusion, absence of any other complications, and to verify the placement of the implant. Scleral depression may enhance visualisation of the implant. Immediate measurement of intraocular pressure (IOP) may be performed at the discretion of the ophthalmologist.
Following the injection, patients should be monitored for change in intraocular pressure and for endophthalmitis. Monitoring may consist of a check for perfusion of the optic nerve head immediately after the injection, tonometry within 30 minutes following the injection, and biomicroscopy between two and seven days following the injection. Patients should be instructed to report without delay any symptoms suggestive of endophthalmitis.

By using PrescriberAI, you agree to the AI Terms of Use.
Yutiq prescribing information
INDICATIONS AND USAGE
YUTIQ ® (fluocinolone acetonide intravitreal implant) 0.18 mg is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye.
DOSAGE AND ADMINISTRATION
General Dosing Information
For ophthalmic intravitreal injection.
Administration
The intravitreal injection procedure should be carried out under aseptic conditions, which include use of sterile gloves, a sterile drape, a sterile caliper, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection.
The injection procedure for YUTIQ is as follows:
- Just prior to injection, administer topical and/or subconjunctival anesthesia at the injection site (inferotemporal quadrant recommended).
- Administer 2-3 drops of a broad-spectrum microbicide into the lower fornix. The lids may be scrubbed with cotton-tipped applicators soaked with a broad-spectrum microbicide. Place a sterile lid speculum. Have the patient look up and apply additional microbicide solution to the injection site. Allow 30-60 seconds for the topical antiseptic to dry prior to injection of YUTIQ.
- Optimal placement of YUTIQ is inferior to the optic disc and posterior to the equator of the eye. Measure 4 millimeters inferotemporal from the limbus with the aid of callipers for point of entry into the sclera.
- Using sterile procedure, open the sterile foil pouch containing YUTIQ.
- Remove the YUTIQ applicator from the sterile pouch by grasping the barrel of the applicator; do not grasp the plunger.
- Remove the black plunger stop from the plunger.
- Carefully remove the protective cap from the needle and inspect the needle tip to ensure it is not bent.
- Remove the trombone wire from the distal end of the needle. Prior to injection, keep the applicator tip above the horizontal plane to ensure that the YUTIQ implant does not fall out of the applicator.
- Gently displace the conjunctiva so that after withdrawing the needle, the conjunctival and scleral needle entry sites will not align. Care should be taken to avoid contact between the needle and the lid margin or lashes.
- Insert the needle through the conjunctiva and sclera up to the positive stop of the applicator.
- Depress the plunger at the back of the applicator fully to deliver the YUTIQ implant into the back of the eye.
- Remove the YUTIQ applicator from the eye and discard in biohazard sharps container.
- Remove the lid speculum and perform indirect ophthalmoscopy to verify adequate central retinal artery perfusion, absence of any other complications, and to verify the placement of the implant. Scleral depression may enhance visualisation of the implant. Immediate measurement of intraocular pressure (IOP) may be performed at the discretion of the ophthalmologist.
Following the injection, patients should be monitored for change in intraocular pressure and for endophthalmitis. Monitoring may consist of a check for perfusion of the optic nerve head immediately after the injection, tonometry within 30 minutes following the injection, and biomicroscopy between two and seven days following the injection. Patients should be instructed to report without delay any symptoms suggestive of endophthalmitis.
DOSAGE FORMS AND STRENGTHS
YUTIQ is a non-bioerodible intravitreal implant in a drug delivery system containing 0.18 mg fluocinolone acetonide, designed to release fluocinolone acetonide at an initial rate of 0.25 mcg/day, and lasting 36 months.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Adequate and well-controlled studies with YUTIQ have not been conducted in pregnant women to inform drug associated risk. Animal reproduction studies have not been conducted with YUTIQ. It is not known whether YUTIQ can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. YUTIQ should be given to a pregnant woman only if the potential benefit justifies the potential risk to the fetus.
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Lactation
Risk Summary
Systemically administered corticosteroids are present in human milk and can suppress growth, interfere with endogenous corticosteroid production. Clinical or nonclinical lactation studies have not been conducted with YUTIQ. It is not known whether intravitreal treatment with YUTIQ could result in sufficient systemic absorption to produce detectable quantities of fluocinolone acetonide in human milk, or affect breastfed infants or milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother's clinical need for YUTIQ and any potential adverse effects on the breastfed child from YUTIQ.
Pediatric Use
Safety and effectiveness of YUTIQ in pediatric patients have not been established.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
CONTRAINDICATIONS
Ocular or Periocular Infections
YUTIQ is contraindicated in patients with active or suspected ocular or periocular infections including most viral disease of the cornea and conjunctiva including active epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, mycobacterial infections and fungal diseases.
Hypersensitivity
YUTIQ is contraindicated in patients with known hypersensitivity to any components of this product.
WARNINGS AND PRECAUTIONS
- Intravitreal injections have been associated with endophthalmitis, eye inflammation, increased intraocular pressure, and retinal detachments. Patients should be monitored following the injection. (5.1 )
- Use of corticosteroids may produce posterior subcapsular cataracts, increased intraocular pressure, glaucoma, and may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses. (5.2 )
- The implant may migrate into the anterior chamber if the posterior lens capsule is not intact. (5.3 )
Intravitreal Injection-related Effects
Intravitreal injections, including those with YUTIQ, have been associated with endophthalmitis, eye inflammation, increased or decreased intraocular pressure, and choroidal or retinal detachments. Hypotony has been observed within 24 hours of injection and has resolved within 2 weeks. Patients should be monitored following the intravitreal injection [see Patient Counseling Information (17 )] .
Steroid-related Effects
Use of corticosteroids including YUTIQ may produce posterior subcapsular cataracts, increased intraocular pressure and glaucoma. Use of corticosteroids may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses.
Corticosteroids are not recommended to be used in patients with a history of ocular herpes simplex because of the potential for reactivation of the viral infection.
Risk of Implant Migration
Patients in whom the posterior capsule of the lens is absent or has a tear are at risk of implant migration into the anterior chamber.
ADVERSE REACTIONS
In controlled studies, the most common adverse reactions reported were cataract development and increases in intraocular pressure. (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact EyePoint Pharmaceuticals US, Inc. at 1-833-EYEPOINT (1-833-393-7646) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions associated with ophthalmic steroids including YUTIQ include cataract formation and subsequent cataract surgery, elevated intraocular pressure, which may be associated with optic nerve damage, visual acuity and field defects, secondary ocular infection from pathogens including herpes simplex, and perforation of the globe where there is thinning of the cornea or sclera.
Studies 1 and 2 were multicenter, randomized, sham injection-controlled, masked trials in which patients with non-infectious uveitis affecting the posterior segment of the eye were treated once with either YUTIQ or sham injection, and then received standard care for the duration of the study. Study 3 was a multicenter, randomized, masked trial in which patients with non-infectious uveitis affecting the posterior segment of the eye were all treated once with YUTIQ, administered by one of two different applicators, and then received standard care for the duration of the study.
Table 1 summarizes data available from studies 1, 2 and 3 through 12 months for study eyes treated with YUTIQ (n=226) or sham injection (n=94). The most common ocular (study eye) and non-ocular adverse reactions are shown in Table 1 and Table 2 .
| Ocular | ||
|---|---|---|
| ADVERSE REACTIONS | YUTIQ (N=226 Eyes) n (%) | Sham Injection (N=94 Eyes) n (%) |
1. Includes cataract, cataract subcapsular and lenticular opacities in study eyes that were phakic at baseline. 113 of the 226 YUTIQ study eyes were phakic at baseline; 56 of 94 sham-controlled study eyes were phakic at baseline. | ||
| Cataract 1 | 63/113 (56%) | 13/56 (23%) |
| Visual Acuity Reduced | 33 ( 15%) | 11 (12%) |
| Macular Edema | 25 ( 11%) | 33 (35%) |
| Uveitis | 22 ( 10%) | 33 (35%) |
| Conjunctival Hemorrhage | 17 ( 8%) | 5 ( 5%) |
| Eye Pain | 17 ( 8%) | 12 (13%) |
| Hypotony Of Eye | 16 ( 7%) | 1 ( 1%) |
| Anterior Chamber Inflammation | 12 ( 5%) | 6 ( 6%) |
| Dry Eye | 10 ( 4%) | 3 ( 3%) |
| Vitreous Opacities | 9 ( 4%) | 8 ( 9%) |
| Conjunctivitis | 9 ( 4%) | 5 ( 5%) |
| Posterior Capsule Opacification | 8 ( 4%) | 3 ( 3%) |
| Ocular Hyperemia | 8 ( 4%) | 7 ( 7%) |
| Vitreous Haze | 7 ( 3%) | 4 ( 4%) |
| Foreign Body Sensation In Eyes | 7 ( 3%) | 2 ( 2%) |
| Vitritis | 6 ( 3%) | 8 ( 9%) |
| Vitreous Floaters | 6 ( 3%) | 5 ( 5%) |
| Eye Pruritus | 6 ( 3%) | 5 ( 5%) |
| Conjunctival Hyperemia | 5 ( 2%) | 2 ( 2%) |
| Ocular Discomfort | 5 ( 2%) | 1 ( 1%) |
| Macular Fibrosis | 5 ( 2%) | 2 ( 2%) |
| Glaucoma | 4 ( 2%) | 1 ( 1%) |
| Photopsia | 4 ( 2%) | 2 ( 2%) |
| Vitreous Hemorrhage | 4 ( 2%) | 0 |
| Iridocyclitis | 3 ( 1%) | 7 ( 7%) |
| Eye Inflammation | 3 ( 1%) | 2 ( 2%) |
| Choroiditis | 3 ( 1%) | 1 ( 1%) |
| Eye Irritation | 3 ( 1%) | 1 ( 1%) |
| Visual Field Defect | 3 ( 1%) | 0 |
| Lacrimation Increased | 3 ( 1%) | 0 |
| Non-ocular | ||
| ADVERSE REACTIONS | YUTIQ (N=214 Patients) n (%) | Sham Injection (N=94 Patients) n (%) |
| Nasopharyngitis | 10 ( 5%) | 5 ( 5%) |
| Hypertension | 6 ( 3%) | 1 ( 1%) |
| Arthralgia | 5 ( 2%) | 1 ( 1%) |
| ADVERSE REACTIONS | YUTIQ (N=226 Eyes) n (%) | Sham (N=94 Eyes) n (%) |
|---|---|---|
| IOP elevation ≥ 10 mmHg from Baseline | 50 (22%) | 11 (12%) |
| IOP elevation > 30 mmHg | 28 (12%) | 3 (3%) |
| Any IOP-lowering medication | 98 (43%) | 39 (41%) |
| Any surgical intervention for elevated IOP | 5 (2%) | 2 (2%) |
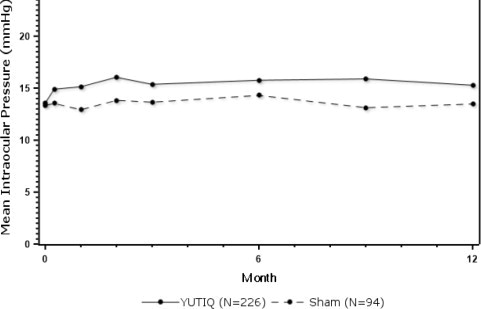
Figure 1: Mean IOP During the Studies
DESCRIPTION
YUTIQ is a sterile non-bioerodible intravitreal implant containing 0.18 mg fluocinolone acetonide in a 36-month sustained-release drug delivery system. YUTIQ is designed to release fluocinolone acetonide at an initial rate of 0.25 mcg/day. YUTIQ is preloaded into a single-dose applicator to facilitate injection of the implant directly into the vitreous. The drug substance is a synthetic corticosteroid, fluocinolone acetonide.
The chemical name for fluocinolone acetonide is (6α,11β, 16α)-6,9-difluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis-(oxy)]-pregna-1,4-diene-3,20-dione. Its chemical structure is:
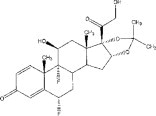
MW 452.50; molecular formula C 24 H 30 F 2 0 6
Fluocinolone acetonide is a white or almost white, microcrystalline powder, practically insoluble in water, soluble in methanol, ethanol, chloroform and acetone, and sparingly soluble in ether.
Each YUTIQ consists of a light brown 3.5mm x 0.37mm implant containing 0.18 mg of the active ingredient fluocinolone acetonide and the following inactive ingredients: polyimide tube, polyvinyl alcohol, silicone adhesive and water for injection.
CLINICAL PHARMACOLOGY
Mechanism of Action
Corticosteroids inhibit inflammatory responses to a variety of inciting agents including multiple inflammatory cytokines. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation.
Corticosteroids are thought to act by inhibition of phospholipase A 2 via induction of inhibitory proteins collectively called lipocortins. It is postulated that these proteins control biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting release of the common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2 .
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been conducted to determine the carcinogenic potential or the effect on fertility of YUTIQ.
Fluocinolone acetonide was not genotoxic in vitro in the Ames test (S. typhimurium and E. coli) and the mouse lymphoma TK assay, or in vivo in the mouse bone marrow micronucleus assay.
CLINICAL STUDIES
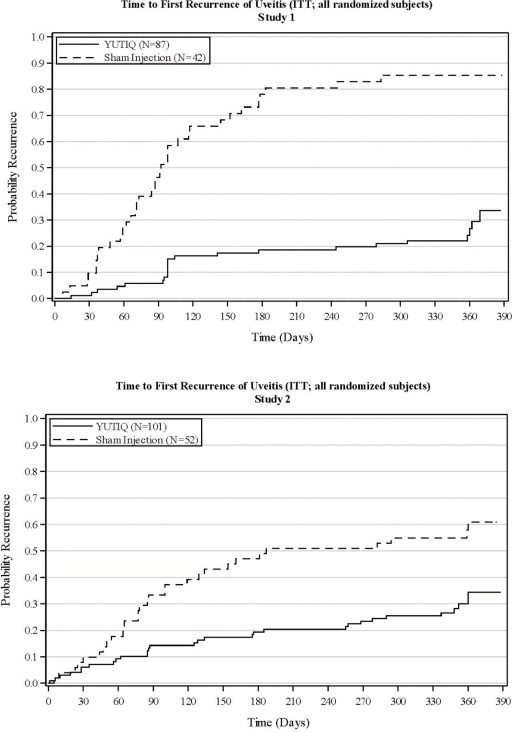
The efficacy of YUTIQ was assessed in two randomized (2:1, YUTIQ: sham-injection), multi-centre, double-masked, parallel-groups studies (NCT #01694186 and #02746991) that enrolled patients with non-infectious uveitis affecting the posterior segment of the eye. The primary efficacy endpoint in both trials was the proportion of patients who experienced a recurrence of uveitis in the study eye within 6 months of follow-up; recurrence was also assessed at 12 months. Recurrence of uveitis was defined as either deterioration in visual acuity, vitreous haze attributable to non-infectious uveitis or the need for rescue medications.
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| YUTIQ N = 87 | Sham N = 42 | YUTIQ N = 101 | Sham N = 52 | ||
| Eyes with recurrence within 6 months, n (%) | 16 (18%) | 33 (79%) | 22 (22%) | 28 (54%) | |
| Difference (95% CI) in recurrence rates | 60% (41%, 73%) | 32% (15%, 48%) | |||
| P-value | < 0.01 | < 0.01 | |||
| Eyes with recurrence within 12 months, n (%) | 24 (28%) | 36 (86%) | 33 (33%) | 31 (60%) | |
| Difference (95% CI) in recurrence rates | 58% (40%, 70%) | 27% (9%, 43%) | |||
Figure 2: Time to First Recurrence of Uveitis (ITT: All Randomized Patients)
HOW SUPPLIED/STORAGE AND HANDLING
YUTIQ ® (fluocinolone acetonide intravitreal implant) 0.18 mg is supplied in a sterile single-dose preloaded applicator with a 25-gauge needle, packaged in a sealed sterile foil pouch inside a sealed Tyvek pouch inside a carton box.
NDC 71879-136-01
Storage: Store at 15° C to 30° C (59° F to 86° F).
Mechanism of Action
Corticosteroids inhibit inflammatory responses to a variety of inciting agents including multiple inflammatory cytokines. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation.
Corticosteroids are thought to act by inhibition of phospholipase A 2 via induction of inhibitory proteins collectively called lipocortins. It is postulated that these proteins control biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting release of the common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2 .