Bleomycin Prescribing Information
It is recommended that Bleomycin for Injection, USP be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate diagnostic and treatment facilities are readily available.
Pulmonary fibrosis is the most severe toxicity associated with bleomycin. The most frequent presentation is pneumonitis occasionally progressing to pulmonary fibrosis. Its occurrence is higher in elderly patients and in those receiving greater than 400 units total dose, but pulmonary toxicity has been observed in young patients and those treated with low doses.
A severe idiosyncratic reaction consisting of hypotension, mental confusion, fever, chills, and wheezing has been reported in approximately 1% of lymphoma patients treated with bleomycin.
Bleomycin for Injection, USP should be considered a palliative treatment. It has been shown to be useful in the management of the following neoplasms either as a single agent or in proven combinations with other approved chemotherapeutic agents:
Bleomycin for Injection, USP has also been shown to be useful in the management of:
The following dose schedule is recommended:
2) given intravenously, intramuscularly, or subcutaneously weekly or twice weekly.
2) given intravenously, intramuscularly, or subcutaneously weekly or twice weekly. After a 50% response, a maintenance dose of 1 unit daily or 5 units weekly intravenously or intramuscularly should be given.
Pulmonary toxicity of Bleomycin for Injection, USP appears to be dose-related with a striking increase when the total dose is over 400 units. Total doses over 400 units should be given with great caution.
Improvement of Hodgkin’s disease and testicular tumors is prompt and noted within 2 weeks. If no improvement is seen by this time, improvement is unlikely. Squamous cell cancers respond more slowly, sometimes requiring as long as 3 weeks before any improvement is noted.
Sixty units of Bleomycin for Injection are dissolved in 50 to 100 mL Sodium Chloride for Injection, 0.9%, USP, and administered through a thoracostomy tube following drainage of excess pleural fluid and confirmation of complete lung expansion. The literature suggests that successful pleurodesis is, in part, dependent upon complete drainage of the pleural fluid and re-establishment of negative intrapleural pressure prior to instillation of a sclerosing agent. Therefore, the amount of drainage from the chest tube should be as minimal as possible prior to instillation of Bleomycin for Injection. Although there is no conclusive evidence to support this contention, it is generally accepted that chest tube drainage should be less than 100 mL in a 24-hour period prior to sclerosis. However, Bleomycin for Injection instillation may be appropriate when drainage is between 100 to 300 mL under clinical conditions that necessitate sclerosis therapy. The thoracostomy tube is clamped after Bleomycin for Injection instillation. The patient is moved from the supine to the left and right lateral positions several times during the next four hours. The clamp is then removed and suction re-established. The amount of time the chest tube remains in place following sclerosis is dictated by the clinical situation.
The intrapleural injection of topical anesthetics or systemic narcotic analgesia is generally not required.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
The following dosing reductions are proposed for patients with creatinine clearance (CrCL) values of less than 50 mL/min:
| Patient CrCL (mL/min) | Bleomycin for Injection, USP Dose (%) |
| 50 and above | 100 |
| 40 to 50 | 70 |
| 30 to 40 | 60 |
| 20 to 30 | 55 |
| 10 to 20 | 45 |
| 5 to 10 | 40 |
CrCL can be estimated from the individual patient’s measured serum creatinine (Scr) values using the Cockcroft and Gault formula:
Males CrCL = [weight x (140 – Age)]/(72 x Scr)
Females CrCL = 0.85 x [weight x (140 – Age)]/(72 x Scr)
Where CrCL in mL/min/1.73 m
2, weight in kg, age in years, and Scr in mg/dL.
Bleomycin for Injection is contraindicated in patients who have demonstrated a hypersensitive or an idiosyncratic reaction to it.
Vascular toxicities coincident with the use of bleomycin in combination with other antineoplastic agents have been reported. The events are clinically heterogeneous and may include myocardial infarction, cerebrovascular accident, thrombotic microangiopathy (HUS), or cerebral arteritis. Various mechanisms have been proposed for these vascular complications. There are also reports of Raynaud’s phenomenon occurring in patients treated with bleomycin in combination with vinblastine with or without cisplatin or, in a few cases, with bleomycin as a single agent. It is currently unknown if the cause of Raynaud’s phenomenon in these cases is the disease, underlying vascular compromise, bleomycin, vinblastine, hypomagnesemia, or a combination of any of these factors.
Fever, chills, and vomiting have been reported. Anorexia and weight loss have been reported and may persist long after termination of this medication. Pain at tumor site, phlebitis, and other local reactions have been reported.
Malaise has been reported.
Bleomycin for Injection, USP is a mixture of cytotoxic glycopeptide antibiotics isolated from a strain of
Bleomycin for Injection, USP is provided as a sterile lyophilized powder for reconstitution containing 15 units per vial and 30 units per vial, which are intended for intramuscular, intravenous, subcutaneous or intrapleural administration.
Its chemical name is N’-[3-(dimethylsulphonio)propyl]bleomycin-amide (bleomycin A
2) and N’-[4-(guaniodobutyl)]bleomycin-amide (bleomycin B
2).
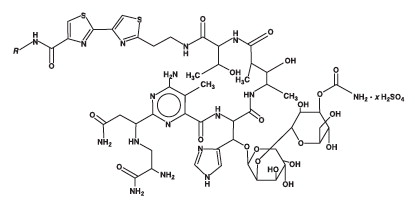
(Main component: Bleomycin A
2, in which
3]
2S
+CH
2CH
2CH
2-)