Brompheniramine Maleate, Pseudoephedrine Hydrochloride, Dextromethorphan Hydrobromide
(Brompheniramine Maleate, Pseudoephedrine Hydrochloride, And Dextromethorphan Hydrobromide)Brompheniramine Maleate, Pseudoephedrine Hydrochloride, Dextromethorphan Hydrobromide Prescribing Information
Brompheniramine Maleate, Pseudoephedrine Hydrochloride, and Dextromethorphan Hydrobromide Syrup is indicated for relief of coughs and upper respiratory symptoms, including nasal congestion, associated with allergy or the common cold.
Adults and pediatric patients 12 years of age and over: 10 mL (2 teaspoonfuls) every 4 hours. Children 6 to under 12 years of age: 5 mL (1 teaspoonful) every 4 hours. Children 2 to under 6 years of age: 2.5 mL (½ teaspoonful) every 4 hours. Infants 6 months to under 2 years of age: Dosage to be established by a physician.
Do not exceed 6 doses during a 24-hour period.
Hypersensitivity to any of the ingredients. Do not use in the newborn, in premature infants, in nursing mothers, or in patients with severe hypertension or severe coronary artery disease. Do not use dextromethorphan in patients receiving monoamine oxidase inhibitors (MAOIs) (see
Hyperpyrexia, hypotension, and death have been reported coincident with the coadministration of MAOIs and products containing dextromethorphan. In addition, MAOIs prolong and intensify the anticholinergic (drying) effects of antihistamines and may enhance the effect of pseudoephedrine. Concomitant administration of Brompheniramine Maleate, Pseudoephedrine Hydrochloride, and Dextromethorphan Hydrobromide Syrup and MAOIs should be avoided (see
Antihistamines have additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, antianxiety agents, etc.).
Sympathomimetic may reduce the effects of antihypertensive drugs.
Antihistamines should not be used to treat lower respiratory tract conditions including asthma.
The most frequent adverse reactions to Brompheniramine Maleate, Pseudoephedrine Hydrochloride, and Dextromethorphan Hydrobromide Syrup are: sedation; dryness of mouth, nose and throat; thickening of bronchial secretions; dizziness. Other adverse reactions may include:
Hyperpyrexia, hypotension, and death have been reported coincident with the coadministration of MAOIs and products containing dextromethorphan. In addition, MAOIs prolong and intensify the anticholinergic (drying) effects of antihistamines and may enhance the effect of pseudoephedrine. Concomitant administration of Brompheniramine Maleate, Pseudoephedrine Hydrochloride, and Dextromethorphan Hydrobromide Syrup and MAOIs should be avoided (see
Brompheniramine Maleate, Pseudoephedrine Hydrochloride, and Dextromethorphan Hydrobromide Syrup is a clear, colorless, sugar free syrup.
Brompheniramine Maleate, USP . . . . . . . . 2 mg
Pseudoephedrine Hydrochloride, USP . . . . . 30 mg
Dextromethorphan Hydrobromide, USP . . . 10 mg
Inactive Ingredients: citric acid anhydrous, glycerin, methylparaben, propylene glycol, purified water, sodium benzoate, sodium citrate dihydrate, and sucralose.
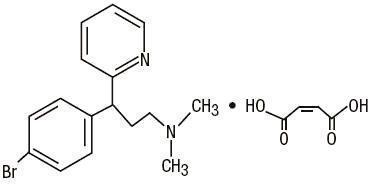
C
16H
19BrN
2•C
4H
4O
4 M.W. 435.31
Brompheniramine Maleate, USP
(±)-2-
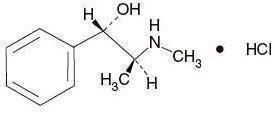
C
10H
15NO•HCl M.W. 201.69
Pseudoephedrine Hydrochloride, USP
(+)-Pseudoephedrine hydrochloride
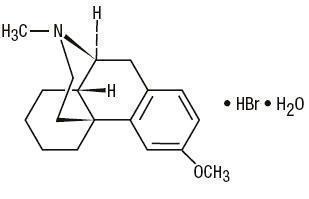
C
18H
25NO•HBr•H
2O M.W. 370.32
Dextromethorphan Hydrobromide, USP
3-Methoxy-17-methyl-9α, 13α, 14α -morphinan hydrobromide monohydrate
Antihistamine/ Nasal Decongestant/ Antitussive syrup for oral administration.