Calcipotriene
Calcipotriene Prescribing Information
Calcipotriene ointment, 0.005%, is indicated for the treatment of plaque psoriasis in adults. The safety and effectiveness of topical calcipotriene in dermatoses other than psoriasis have not been established.
Apply a thin layer of calcipotriene ointment once or twice daily and rub in gently and completely.
Calcipotriene is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation. It should not be used by patients with demonstrated hypercalcemia or evidence of vitamin D toxicity. Calcipotriene should not be used on the face.
In controlled clinical trials, the most frequent adverse experiences reported for calcipotriene were burning, itching and skin irritation, which occurred in approximately 10 to 15% of patients. Erythema, dry skin, peeling, rash, dermatitis, worsening of psoriasis including development of facial/scalp psoriasis were reported in 1 to 10% of patients. Other experiences reported in less than 1% of patients included skin atrophy, hyperpigmentation, hypercalcemia, and folliculitis. Once daily dosing has not been shown to be superior in safety to twice daily dosing.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions associated with the use of calcipotriene ointment have been identified post-approval: contact dermatitis, including allergic contact dermatitis.
Calcipotriene Ointment USP, 0.005% contains the compound calcipotriene, USP a synthetic vitamin D3 derivative for topical dermatological use.
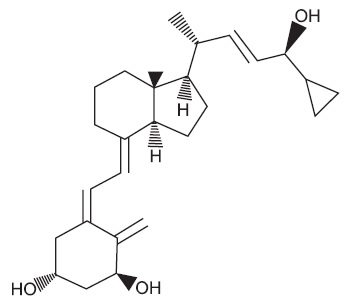
Chemically, calcipotriene, USP is24-cyclopropyl-(1α,3β,5Z,7E,22E,24S)-9,10-Secochola-5,7,10(19),22-tetraene-1,3,24-trioI; with the empirical formula C27H40O3, a molecular weight of 412.62, and the following structural formula:
Calcipotriene, USP is a white or almost white, crystalline powder. Calcipotriene Ointment USP, 0.005% contains calcipotriene, USP 50 mcg/g in an ointment base of disodium phosphate dihydrate, edetate disodium, mineral oil, white petrolatum, propylene glycol,
In humans, the natural supply of vitamin D depends mainly on exposure to the ultraviolet rays of the sun for conversion of 7-dehydrocholesterol to vitamin D3 (cholecalciferol) in the skin. Calcipotriene is a synthetic analog of vitamin D3.
Clinical studies with radiolabelled calcipotriene ointment indicate that approximately 6% (± 3%, SD) of the applied dose of calcipotriene is absorbed systemically when the ointment is applied topically to psoriasis plaques or 5% (± 2.6%, SD) when applied to normal skin, and much of the absorbed active is converted to inactive metabolites within 24 hours of application.
Vitamin D and its metabolites are transported in the blood, bound to specific plasma proteins. The active form of the vitamin, 1,25-dihydroxy vitamin D3 (calcitriol), is known to be recycled via the liver and excreted in the bile. Calcipotriene metabolism following systemic uptake is rapid, and occurs via a similar pathway to the natural hormone. The primary metabolites are much less potent than the parent compound.
There is evidence that maternal 1,25-dihydroxy vitamin D3 (calcitriol) may enter the fetal circulation, but it is not known whether it is excreted in human milk. The systemic disposition of calcipotriene is expected to be similar to that of the naturally occurring vitamin.