Cefadroxil
Cefadroxil Prescribing Information
Cefadroxil for oral suspension USP is indicated for the treatment of patients with infection caused by susceptible strains of the designated organisms in the following diseases:
Urinary tract infections caused by
Skin and skin structure infections caused by staphylococci and/or streptococci.
Pharyngitis and/or tonsillitis caused by
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil for oral suspension and other antibacterial drugs, cefadroxil for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Cefadroxil for oral suspension is acid-stable and may be administered orally without regard to meals. Administration with food may be helpful in diminishing potential gastrointestinal complaints occasionally associated with oral cephalosporin therapy.
Cefadroxil monohydrate is contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment (see
BEFORE THERAPY WITH CEFADROXIL MONOHYDRATE IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFADROXIL, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-SENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY.
IF AN ALLERGIC REACTION TO CEFADROXIL MONOHYDRATE OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against
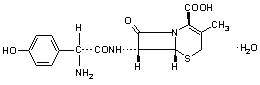
Cefadroxil monohydrate is a semisynthetic cephalosporin antibiotic intended for oral administration. It is a white to yellowish-white crystalline powder. It is soluble in water and it is acid-stable. It is chemically designated as 5-Thia-1-azabicyclo[4.2.O]oct-2-ene-2-carboxylic acid, 7-[[amino(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-, monohydrate, [6R-[6(,7((R*)]]-. It has the formula C16H17N3O5S•H2O and the molecular weight of 381.40. It has the following structural formula:
Cefadroxil for oral suspension USP contains cefadroxil monohydrate USP. After reconstitution, each 5 mL contains cefadroxil monohydrate USP equivalent to 250 mg or 500 mg of cefadroxil. In addition, cefadroxil for oral suspension USP contains the following inactive ingredients: colloidal silicon dioxide, FD&C Yellow No. 6, powder flavor orange, powder flavor pineapple, sodium benzoate, sucrose, and xanthan gum.
Cefadroxil for oral suspension USP is a light orange colored powder, forming orange colored suspension on constitution.
Cefadroxil monohydrate is rapidly absorbed after oral administration. Following single doses of 500 mg and 1000 mg, average peak serum concentrations were approximately 16 and 28 mcg/mL, respectively. Measurable levels were present 12 hours after administration. Over 90% of the drug is excreted unchanged in the urine within 24 hours. Peak urine concentrations are approximately 1800 mcg/mL during the period following a single 500-mg oral dose. Increases in dosage generally produce a proportionate increase in cefadroxil monohydrate urinary concentration. The urine antibiotic concentration, following a 1-g dose, was maintained well above the MIC of susceptible urinary pathogens for 20 to 22 hours.