Ciclopirox Olamine
Ciclopirox Olamine Prescribing Information
Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris and tinea corporis due to
Gently massage Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) into the affected and surrounding skin areas twice daily, in the morning and evening. Clinical improvement with relief of pruritus and other symptoms usually occurs within the first week of treatment. If a patient shows no clinical improvement after four weeks of treatment with Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) the diagnosis should be redetermined. Patients with tinea versicolor usually exhibit clinical and mycological clearing after two weeks of treatment.
Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) is contraindicated in individuals who have shown hypersensitivity to any of its components.
In the controlled clinical trial with 89 patients using ciclopirox olamine topical suspension and 89 patients using the vehicle, the incidence of adverse reactions was low. Those considered possibly related to treatment or occurring in more than one patient were pruritus, which occurred in two patients using ciclopirox olamine topical suspension and one patient using the suspension vehicle, and burning, which occurred in one patient using ciclopirox olamine topical suspension.
Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) is for topical use.
Each gram of Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) contains 7.70 mg of ciclopirox (as ciclopirox olamine) in a water miscible suspension base consisting of benzyl alcohol (1% as a preservative), cetyl alcohol, lactic acid, light mineral oil, myristyl alcohol, octyldodecanol, polysorbate 60, purified water, sorbitan monostearate, and stearyl alcohol.
Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) contains a synthetic, broad-spectrum, antifungal agent ciclopirox (as ciclopirox olamine).
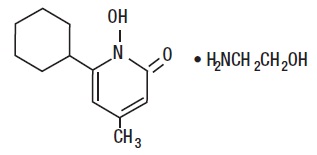
The chemical name is 6-cyclohexyl-1-hydroxy-4-methyl-2(1
The CAS Registry Number is 41621-49-2.
Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) has a pH of 7. The chemical structure is:
Ciclopirox is a hydroxypyridone antifungal agent that acts by chelation of polyvalent cations (Fe3+ or Al3+), resulting in the inhibition of the metal-dependent enzymes that are responsible for the degradation of peroxides within the fungal cell.
Pharmacokinetic studies in men with radiolabeled ciclopirox solution in polyethylene glycol 400, showed an average of 1.3% absorption of the dose when it was applied topically to 750 cm2 on the back followed by occlusion for 6 hours.
The biological half-life was 1.7 hours and excretion occurred via the kidney. Two days after application only 0.01% of the dose applied could be found in the urine. Fecal excretion was negligible. Autoradiographic studies with human cadaver skin showed that ciclopirox penetrates into the hair and through the epidermis and hair follicles into the sebaceous glands and dermis, while a portion of the drug remains in the stratum corneum.