Cysto - Conray Ii - Iothalamate Meglumine injection Prescribing Information
Cysto-Conray II is indicated for use in retrograde cystography and cystourethrography.
Unless contraindicated, an appropriate laxative is given the night before the examination.
See
Irritation of the bladder or ureter, common to some degree to all contrast media administered for retrograde urographic procedures, may occasionally occur.
As with all contrast media, intravasation may lead to hypersensitivity reactions such as a sense of warmth, flushing, sneezing, sweating, chills, fever, urticaria, laryngeal edema, bronchospasm, hypertension, hypotension, cardiac arrhythmias and cardiac arrest.
Adverse reactions associated with procedural technique include injury to the urethra, bladder, ureter, and introduction of infection.
In the event of serious or anaphylactoid reactions, it should be kept in mind that the reactions known to occur with intravenous administration of radiopaque contrast materials are possible.
Cysto-Conray II is a sterile aqueous solution intended for instillation as a diagnostic radiopaque medium. Cysto-Conray II contains 17.2% w/v iothalamate meglumine which is 1-Deoxy-1-(methylamino)-D-glucitol 5-acetamido-2,4,6-triiodo-N-methylisophthalamate (salt) and has the following structural formula:
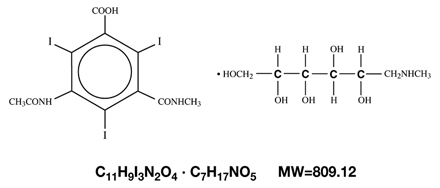
Each milliliter of Cysto-Conray II contains 172 mg of iothalamate meglumine, equivalent to 81 mg (8.1% w/v) of organically bound iodine, 0.110 mg edetate calcium disodium as a stabilizer and 0.115 mg of monobasic sodium phosphate as a buffer.
Cysto-Conray II is hypertonic under conditions of use and is supplied in containers from which the air has been displaced by nitrogen. The pH of Cysto-Conray II is
6.6 to 7.6.