Doxepin
Doxepin Prescribing Information
Doxepin tablets are indicated for the treatment of insomnia characterized by difficulty with sleep maintenance. The clinical trials performed in support of efficacy were up to 3 months in duration.
The dose of doxepin tablets should be individualized.
Doxepin Tablets are available containing 3.39 mg or 6.78 mg of doxepin hydrochloride, USP equivalent to 3 mg or 6 mg of doxepin, respectively. Doxepin tablets are not scored.
• The 3 mg tablets are light blue, round, biconvex, beveled edge tablet debossed with
• The 6 mg tablets are blue, round, biconvex, beveled edge tablet debossed with
The following serious adverse reactions are discussed in greater detail in other sections of labeling:
• Abnormal thinking and behavioral changes [
Complex behaviors such as “sleep-driving” (i.e., driving while not fully awake after ingestion of a hypnotic, with amnesia for the event) have been reported with hypnotics. These events can occur in hypnotic-naive as well as in hypnotic-experienced persons. Although behaviors such as “sleep-driving” may occur with hypnotics alone at therapeutic doses, the use of alcohol and other CNS depressants with hypnotics appears to increase the risk of such behaviors, as does the use of hypnotics at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of doxepin tablets should be strongly considered for patients who report a “sleep-driving” episode. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a hypnotic. As with “sleep-driving”, patients usually do not remember these events. Amnesia, anxiety and other neuro-psychiatric symptoms may occur unpredictably.
• Suicide risk and worsening of depression [
In primarily depressed patients, worsening of depression, including suicidal thoughts and actions (including completed suicides), has been reported in association with the use of hypnotics.
Doxepin, the active ingredient in doxepin tablets, is an antidepressant at doses 10- to 100-fold higher than in doxepin tablets. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Risk from the lower dose of doxepin in doxepin tablets can not be excluded.
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
In primarily depressed patients, worsening of depression, including suicidal thoughts and actions (including completed suicides), has been reported in association with the use of hypnotics.
Doxepin, the active ingredient in doxepin tablets, is an antidepressant at doses 10- to 100-fold higher than in doxepin tablets. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Risk from the lower dose of doxepin in doxepin tablets can not be excluded.
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
• CNS Depressant effects [
After taking doxepin tablets, patients should confine their activities to those necessary to prepare for bed. Patients should avoid engaging in hazardous activities, such as operating a motor vehicle or heavy machinery, at night after taking doxepin tablets, and should be cautioned about potential impairment in the performance of such activities that may occur the day following ingestion.
When taken with doxepin tablets, the sedative effects of alcoholic beverages, sedating antihistamines, and other CNS depressants may be potentiated [
Doxepin Tablets are available in 3 mg and 6 mg strengths for oral administration. Each tablet contains 3.39 mg or 6.78 mg doxepin hydrochloride, equivalent to 3 mg and 6 mg of doxepin, respectively.
Chemically, doxepin hydrochloride is an (E) and (Z) geometric, isomeric mixture of 1-propanamine, 3-dibenz[
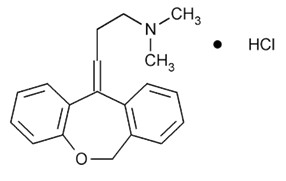
Doxepin hydrochloride, USP is a white crystalline powder, with a slight amine-like odor, that is readily soluble in water. It has a molecular weight of 315.84 and molecular formula of C
19H
21NO•HCl.
Each doxepin tablet includes the following inactive ingredients: colloidal silicon dioxide, FD&C Blue No. 1 Aluminum Lake, magnesium stearate and microcrystalline cellulose.
The mechanism of action of doxepin in sleep maintenance is unclear; however, doxepin’s effect could be mediated through antagonism of the H1 receptor