Doxercalciferol
Doxercalciferol Prescribing Information
- Doxercalciferol injection is indicated for the treatment of secondary hyperparathyroidism in adult patients with CKD on dialysis.
Injection: Sterile, clear and colorless aqueous solution available as follows:
- 2 mcg/mL single-dose vial
- 4 mcg/2 mL (2 mcg/mL) single-dose vial
Doxercalciferol is contraindicated in patients with:
- Hypercalcemia [see Warnings and Precautions (5.1)]
- Vitamin D toxicity [see Warnings and Precautions (5.1)]
- Known hypersensitivity to doxercalciferol or any of the inactive ingredients of doxercalciferol injection; serious hypersensitivity reactions including anaphylaxis and angioedema have been reported [see Warnings and Precautions (5.3), Adverse Reactions (6.2)].
The following adverse reactions are discussed in greater detail in another section of the label:
- Hypercalcemia [see Warnings and Precautions (5.1)]
- Serious Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Adynamic Bone Disease [see Warnings and Precautions (5.4)]
Tables 3 include clinically significant drug interactions with doxercalciferol injection.
Drugs that May Increase the Risk of Hypercalcemia | |
Clinical Impact | Concomitant administration of high doses of calcium-containing preparations or other vitamin D compounds may increase the risk of hypercalcemia. Thiazide diuretics are known to induce hypercalcemia by reducing excretion of calcium in the urine. |
Examples | Calcium-containing products, other vitamin D compounds or thiazide diuretics |
Intervention | Monitor serum calcium concentrations more frequently and adjust doxercalciferol dose as needed [see Warnings and Precautions (5.1)]. |
Digitalis Compounds | |
Clinical Impact | Doxercalciferol can cause hypercalcemia which can potentiate the risk of digitalis toxicity. |
Intervention | Monitor patients for signs and symptoms of digitalis toxicity and increase frequency of serum calcium monitoring when initiating or adjusting the dose of doxercalciferol in patients receiving digitalis compounds [see Warnings and Precautions (5.2)]. |
Cytochrome P450 Inhibitors | |
Clinical Impact | Doxercalciferol is activated by CYP 27 in the liver. Cytochrome P450 inhibitors may inhibit the 25-hydroxylation of doxercalciferol and thus reduce the formation of active doxercalciferol moiety [see Clinical Pharmacology (12.3)]. |
Examples | Ketoconazole and erythromycin |
Intervention | If a patient initiates or discontinues therapy with a cytochrome P450 inhibitor, dose adjustment of doxercalciferol may be necessary. Monitor intact PTH and serum calcium concentrations closely. |
Enzyme Inducers | |
Clinical Impact | Doxercalciferol is activated by CYP 27 in the liver. Enzyme inducers may affect the 25-hydroxylation of doxercalciferol [see Clinical Pharmacology (12.3)]. |
Examples | Glutethimide and phenobarbital |
Intervention | If a patient initiates or discontinues therapy with an enzyme inducer, dose adjustment of doxercalciferol may be necessary. Monitor intact PTH and serum calcium concentrations closely. |
Magnesium-containing Products | |
Clinical Impact | Concomitant administration of doxercalciferol and high doses of magnesium-containing products may increase the risk of hypermagnesemia. |
Examples | Magnesium-containing products such as antacids |
Intervention | Avoid use of magnesium-containing products and doxercalciferol in patients on chronic renal dialysis. |
Doxercalciferol injection contains doxercalciferol, which is a synthetic vitamin D2 analog. Doxercalciferol undergoes metabolic activation
Doxercalciferol, USP is a colorless crystalline compound with a calculated molecular weight of 412.66 and a molecular formula of C28H44O2. It is soluble in oils and organic solvents, but is relatively insoluble in water. Chemically, doxercalciferol is (1α,3β,5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraene-1,3-diol. The structural formula is:
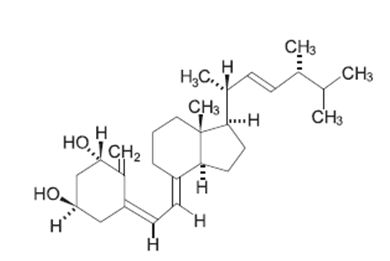
Doxercalciferol injection 1 mL single-dose vials contain 2 mcg/mL of doxercalciferol, USP. Doxercalciferol injection 2 mL single-dose vials contain 4 mcg/2 mL (2 mcg/mL) of doxercalciferol, USP. Each milliliter (mL) of solution contains 2 mcg doxercalciferol, USP and the following inactive ingredients: butylated hydroxytoluene (0.02 mg); disodium edetate (1.1 mg); ethanol, 100% (0.05 mL); polysorbate 20 (10 mg); sodium chloride (1.5 mg); sodium phosphate dibasic, heptahydrate (14.4 mg); and sodium phosphate monobasic, monohydrate (1.8 mg).