Lidocaine
Lidocaine Prescribing Information
Apply lidocaine patch 5% to intact skin to cover the most painful area. Apply the prescribed number of patches (maximum of 3), only once for up to 12 hours within a 24 hour period. Patches may be cut into smaller sizes with scissors prior to removal of the release liner (See
Hands should be washed after the handling of lidocaine patch 5%, and eye contact with lidocaine patch 5% should be avoided. Do not store patch outside the sealed envelope. Apply immediately after removal from the protective envelope. Fold used patches so that the adhesive side sticks to itself and safely discard used patches or pieces of cut patches where children and pets cannot get to them. Lidocaine patch 5% should be kept out of the reach of children.
If irritation or a burning sensation occurs during application, remove the patch(es) and do not reapply until the irritation subsides.
When lidocaine patch 5% is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered.
Lidocaine patch 5% may not stick if it gets wet. Avoid contact with water, such as bathing, swimming or showering.
Lidocaine patch 5% is contraindicated in patients with a known history of sensitivity to local anesthetics of the amide type, or to any other component of the product.
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Class | Examples |
Nitrates/Nitrites | nitric oxide, nitroglycerin, nitroprusside, nitrous oxide |
Local anesthetics | articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine |
Antineoplastic agents | cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase |
Antibiotics | dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides |
Antimalarials | chloroquine, primaquine |
Anticonvulsants | phenobarbital, phenytoin, sodium valproate |
Other drugs | acetaminophen, metoclopramide, quinine, sulfasalazine |
Lidocaine patch 5% is comprised of an adhesive material containing 5% lidocaine, USP, which is applied to a white non-woven polyethylene terephthalate (PET) material backing and covered with a transparent PET release liner. The release liner is removed prior to application to the skin. The size of the patch is 10 cm x 14 cm.
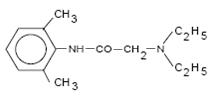
Lidocaine, USP is chemically designated as acetamide, 2-(diethylamino)-N-(2,6- dimethylphenyl), has an octanol:water partition ratio of 43 at pH 7.4, and has the following structure:
Each adhesive patch contains 700 mg of lidocaine, USP (50 mg per gram adhesive) in an aqueous base. It also contains the following inactive ingredients: glycerin, D-sorbitol, propylene glycol, polyvinyl alcohol, urea, sodium polyacrylate, carboxymethylcellulose sodium, gelatin, polyacrylic acid, kaolin, tartaric acid, dihydroxyaluminum aminoacetate, methylparaben, propylparaben, and edetate disodium.
Single-dose treatment with lidocaine patch was compared to treatment with vehicle patch (without lidocaine), and to no treatment (observation only) in a double-blind, crossover clinical trial with 35 post-herpetic neuralgia patients. Pain intensity and pain relief scores were evaluated periodically for 12 hours. Lidocaine patch performed statistically better than vehicle patch in terms of pain intensity from 4 to 12 hours.
Multiple-dose, two-week treatment with lidocaine patch was compared to vehicle patch (without lidocaine) in a double-blind, crossover clinical trial of withdrawal-type design conducted in 32 patients, who were considered as responders to the open-label use of lidocaine patch prior to the study. The constant type of pain was evaluated but not the pain induced by sensory stimuli (dysesthesia). Statistically significant differences favoring lidocaine patch were observed in terms of time to exit from the trial (14 versus 3.8 days at p-value <0.001), daily average pain relief, and patient’s preference of treatment. About half of the patients also took oral medication commonly used in the treatment of post-herpetic neuralgia. The extent of use of concomitant medication was similar in the two treatment groups.
Lidocaine patch 5% is available as the following:
Carton of 30 patches, packaged into individual child-resistant envelopes.
NDC 42291-495-30
Store at 20o to 25oC (68o to 77oF) [See USP Controlled Room Temperature].
For more information, call Teva at 1-888-838-2872.
Manufactured For:
AvKARE
Pulaski, TN 38478
www.avkare.com
Rev. A 4/2022