Lisdexamfetamine Dimesylate Prescribing Information
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Before prescribing lisdexamfetamine dimesylate capsules, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout lisdexamfetamine dimesylate capsules treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction
Lisdexamfetamine dimesylate capsules have a high potential for abuse and misuse. The use of lisdexamfetamine dimesylate capsules exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Lisdexamfetamine dimesylate capsules can be diverted for non-medical use into illicit channels or distribution
Before prescribing lisdexamfetamine dimesylate capsules, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks and proper disposal of any unused drug. Advise patients to store lisdexamfetamine dimesylate capsules in a safe place, preferably locked, and instruct patients to not give lisdexamfetamine dimesylate capsules to anyone else. Throughout lisdexamfetamine dimesylate capsules treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
Lisdexamfetamine Dimesylate Capsules have a high potential for abuse and misuse which can lead to the development of a substance use disorder, including addiction
Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Misuse and abuse of lisdexamfetamine, a prodrug of amphetamine, may cause increased heart rate, respiratory rate, or blood pressure; sweating; dilated pupils; hyperactivity; restlessness; insomnia; decreased appetite; loss of coordination; tremors; flushed skin; vomiting; and/or abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed with CNS stimulants abuse and/or misuse. Misuse and abuse of CNS stimulants, including lisdexamfetamine dimesylate capsules, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
A randomized, double-blind, placebo-control, cross-over, abuse liability study in 38 patients with a history of drug abuse was conducted with single-doses of 50, 100, or 150 mg of lisdexamfetamine dimesylate, 40 mg of immediate-release d-amphetamine sulphate (a controlled II substance), and 200 mg of diethylpropion hydrochloride (a controlled IV substance). Lisdexamfetamine dimesylate 100 mg produced significantly less "Drug Liking Effects" as measured by the Drug Rating Questionnaire-Subject score, compared to d-amphetamine 40 mg; and 150 mg of lisdexamfetamine dimesylate demonstrated similar "Drug-Liking Effects" compared to 40 mg of d-amphetamine and 200 mg of diethylpropion.
Intravenous administration of 50 mg lisdexamfetamine dimesylate to individuals with a history of drug abuse produced positive subjective responses on scales measuring "Drug Liking", "Euphoria", "Amphetamine Effects", and "Benzedrine Effects" that were greater than placebo but less than those produced by an equivalent dose (20 mg) of intravenous d-amphetamine.
Indications and Usage (Lisdexamfetamine dimesylate capsules are indicated for the treatment of:
Lisdexamfetamine dimesylate capsules are a central nervous system (CNS) stimulant indicated for the treatment of :
Limitations of Use :
Limitations of Use:
| 09/2025 |
Warnings and Precautions (Lisdexamfetamine dimesylate capsules are not approved for use and is not recommended in pediatric patients below 6 years of age [see Use in Specific Populations (8.4)]. CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients. In a 4-week, placebo-controlled trial of lisdexamfetamine dimesylate in pediatric patients ages 6 to 12 years old with ADHD, there was a dose-related decrease in weight in the lisdexamfetamine dimesylate groups compared to weight gain in the placebo group. Additionally, in studies of another stimulant, there was slowing of the increase in height [see Adverse Reactions (6.1) ].Closely monitor growth (weight and height) in lisdexamfetamine dimesylate-treated pediatric patients. Patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted. | 09/2025 |
Lisdexamfetamine dimesylate capsules are indicated for the treatment of:
- Attention Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years and older [see]
14.1 Attention Deficit Hyperactivity Disorder (ADHD)Pediatric Patients Ages 6 to 12 Years with ADHDA double-blind, randomized, placebo-controlled, parallel-group study (Study 1) was conducted in pediatric patients ages 6 to 12 years (N=290) who met DSM-IV criteria for ADHD (either the combined type or the hyperactive-impulsive type). Patients were randomized to receive final doses of 30 mg, 50 mg, or 70 mg of lisdexamfetamine dimesylate or placebo once daily in the morning for a total of four weeks of treatment. All patients receiving lisdexamfetamine dimesylate were initiated on 30 mg for the first week of treatment. Patients assigned to the 50 mg and 70 mg dose groups were titrated by 20 mg per week until they achieved their assigned dose. The primary efficacy outcome was change in Total Score from baseline to endpoint in investigator ratings on the ADHD Rating Scale (ADHD-RS), an 18-item questionnaire with a score range of 0-54 points that measures the core symptoms of ADHD which includes both hyperactive/impulsive and inattentive subscales. Endpoint was defined as the last post-randomization treatment week (i.e., Weeks 1 through 4) for which a valid score was obtained. All lisdexamfetamine dimesylate dose groups were superior to placebo in the primary efficacy outcome. Mean effects at all doses were similar; however, the highest dose (70 mg/day) was numerically superior to both lower doses (Study 1 in Table 6). The effects were maintained throughout the day based on parent ratings (Conners' Parent Rating Scale) in the morning (approximately 10 am), afternoon (approximately 2 pm), and early evening (approximately 6 pm).
A double-blind, placebo-controlled, randomized, crossover design, analog classroom study (Study 2) was conducted in pediatric patients ages 6 to 12 years (N=52) who met DSM-IV criteria for ADHD (either the combined type or the hyperactive-impulsive type). Following a 3-week open-label dose optimization with Adderall XR®, patients were randomly assigned to continue their optimized dose of Adderall XR (10 mg, 20 mg, or 30 mg), lisdexamfetamine dimesylate (30 mg, 50 mg, or 70 mg), or placebo once daily in the morning for 1 week each treatment. Efficacy assessments were conducted at 1, 2, 3, 4.5, 6, 8, 10, and 12 hours post-dose using the Swanson, Kotkin, Agler, M.Flynn, and Pelham Deportment scores (SKAMP-DS), a 4-item subscale of the SKAMP with scores ranging from 0 to 24 points that measures deportment problems leading to classroom disruptions. A significant difference in patient behavior, based upon the average of investigator ratings on the SKAMP-DS across the 8 assessments were observed between patients when they received lisdexamfetamine dimesylate compared to patients when they received placebo (Study 2 in Table 6). The drug effect reached statistical significance from hours 2 to 12 post-dose, but was not significant at 1 hour.
A second double-blind, placebo-controlled, randomized, crossover design, analog classroom study (Study 3) was conducted in pediatric patients ages 6 to 12 years (N=129) who met DSM-IV criteria for ADHD (either the combined type or the hyperactive-impulsive type). Following a 4-week open-label dose optimization with lisdexamfetamine dimesylate (30 mg, 50 mg, 70 mg), patients were randomly assigned to continue their optimized dose of lisdexamfetamine dimesylate or placebo once daily in the morning for 1 week each treatment. A significant difference in patient behavior, based upon the average of investigator ratings on the SKAMP-Deportment scores across all 7 assessments conducted at 1.5, 2.5, 5.0, 7.5, 10.0, 12.0, and 13.0 hours post-dose, were observed between patients when they received lisdexamfetamine dimesylate compared to patients when they received placebo (Study 3 in Table 6, Figure 4).
Pediatric Patients Ages 13 to 17 Years with ADHDA double-blind, randomized, placebo-controlled, parallel-group study (Study 4) was conducted in pediatric patients ages 13 to 17 years (N=314) who met DSM-IV criteria for ADHD. In this study, patients were randomized in a 1:1:1:1 ratio to a daily morning dose of lisdexamfetamine dimesylate (30 mg/day, 50 mg/day or 70 mg/day) or placebo for a total of four weeks of treatment. All patients receiving lisdexamfetamine dimesylate were initiated on 30 mg for the first week of treatment. Patients assigned to the 50 mg and 70 mg dose groups were titrated by 20 mg per week until they achieved their assigned dose. The primary efficacy outcome was change in Total Score from baseline to endpoint in investigator ratings on the ADHD Rating Scale (ADHD-RS). Endpoint was defined as the last post-randomization treatment week (i.e., Weeks 1 through 4) for which a valid score was obtained. All lisdexamfetamine dimesylate dose groups were superior to placebo in the primary efficacy outcome (Study 4 in Table 6).
Pediatric Patients Ages 6 to 17 Years: Short-Term Treatment in ADHDA double-blind, randomized, placebo- and active-controlled parallel-group, dose-optimization study (Study 5) was conducted in pediatric patients ages 6 to 17 years (n=336) who met DSM-IV criteria for ADHD. In this eight-week study, patients were randomized to a daily morning dose of lisdexamfetamine dimesylate (30, 50 or 70 mg/day), an active control, or placebo (1:1:1). The study consisted of a Screening and Washout Period (up to 42 days), a 7-week Double-blind Evaluation Period (consisting of a 4-week Dose-Optimization Period followed by a 3-week Dose-Maintenance Period), and a 1-week Washout and Follow-up Period. During the Dose Optimization Period, subjects were titrated until an optimal dose, based on tolerability and investigator's judgment, was reached. Lisdexamfetamine dimesylate showed significantly greater efficacy than placebo. The placebo-adjusted mean reduction from baseline in the ADHD-RS-IV total score was 18.6. Subjects on lisdexamfetamine dimesylate also showed greater improvement on the Clinical Global Impression-Improvement (CGI-I) rating scale compared to subjects on placebo (Study 5 in Table 6).
Pediatric Patients Ages 6 to 17 Years: Maintenance Treatment in ADHDMaintenance of Efficacy Study (Study 6) - A double-blind, placebo-controlled, randomized withdrawal study was conducted in pediatric patients ages 6 to 17 years (N=276) who met the diagnosis of ADHD (DSM-IV criteria). A total of 276 patients were enrolled into the study, 236 patients participated in Study 5 and 40 subjects directly enrolled. Subjects were treated with open-label lisdexamfetamine dimesylate for at least 26 weeks prior to being assessed for entry into the randomized withdrawal period. Eligible patients had to demonstrate treatment response as defined by CGI-S <3 and Total Score on the ADHD-RS ≤22. Patients that maintained treatment response for 2 weeks at the end of the open label treatment period were eligible to be randomized to ongoing treatment with the same dose of lisdexamfetamine dimesylate (N=78) or switched to placebo (N=79) during the double-blind phase. Patients were observed for relapse (treatment failure) during the 6 week double blind phase. A significantly lower proportion of treatment failures occurred among lisdexamfetamine dimesylate subjects (15.8%) compared to placebo (67.5%) at endpoint of the randomized withdrawal period. The endpoint measurement was defined as the last post-randomization treatment week at which a valid ADHD-RS Total Score and CGI-S were observed. Treatment failure was defined as a ≥50% increase (worsening) in the ADHD-RS Total Score and a ≥2-point increase in the CGI-S score compared to scores at entry into the double-blind randomized withdrawal phase. Subjects who withdrew from the randomized withdrawal period and who did not provide efficacy data at their last on-treatment visit were classified as treatment failures (Study 6, Figure 5).
Adults: Short-Term Treatment in ADHDA double-blind, randomized, placebo-controlled, parallel-group study (Study 7) was conducted in adults ages 18 to 55 (N=420) who met DSM-IV criteria for ADHD. In this study, patients were randomized to receive final doses of 30 mg, 50 mg, or 70 mg of lisdexamfetamine dimesylate or placebo for a total of four weeks of treatment. All patients receiving lisdexamfetamine dimesylate were initiated on 30 mg for the first week of treatment. Patients assigned to the 50 mg and 70 mg dose groups were titrated by 20 mg per week until they achieved their assigned dose. The primary efficacy outcome was change in Total Score from baseline to endpoint in investigator ratings on the ADHD Rating Scale (ADHD-RS). Endpoint was defined as the last post-randomization treatment week (i.e., Weeks 1 through 4) for which a valid score was obtained. All lisdexamfetamine dimesylate dose groups were superior to placebo in the primary efficacy outcome (Study 7 in Table 6).
The second study was a multi-center, randomized, double-blind, placebo-controlled, cross-over, modified analog classroom study (Study 8) of lisdexamfetamine dimesylate to simulate a workplace environment in 142 adults ages 18 to 55 who met DSM-IV-TR criteria for ADHD. There was a 4-week open-label, dose optimization phase with lisdexamfetamine dimesylate (30 mg/day, 50 mg/day, or 70 mg/day in the morning). Patients were then randomized to one of two treatment sequences: 1) lisdexamfetamine dimesylate (optimized dose) followed by placebo, each for one week, or 2) placebo followed by lisdexamfetamine dimesylate, each for one week. Efficacy assessments occurred at the end of each week, using the Permanent Product Measure of Performance (PERMP), a skill-adjusted math test that measures attention in ADHD. PERMP total score results from the sum of the number of math problems attempted plus the number of math problems answered correctly. Lisdexamfetamine dimesylate treatment, compared to placebo, resulted in a statistically significant improvement in attention across all post-dose time points, as measured by average PERMP total scores over the course of one assessment day, as well as at each time point measured. The PERMP assessments were administered at pre-dose (-0.5 hours) and at 2, 4, 8,10,12, and 14 hours post-dose (Study 8 in Table 6, Figure 6).
Adults: Maintenance Treatment in ADHDA double-blind, placebo-controlled, randomized withdrawal design study (Study 9) was conducted in adults ages 18 to 55 (N=123) who had a documented diagnosis of ADHD or met DSM-IV criteria for ADHD. At study entry, patients must have had documentation of treatment with lisdexamfetamine dimesylate for a minimum of 6 months and had to demonstrate treatment response as defined by Clinical Global Impression Severity (CGI-S) ≤3 and Total Score on the ADHD-RS <22. ADHD-RS Total Score is a measure of core symptoms of ADHD. The CGI-S score assesses the clinician's impression of the patient's current illness state and ranges from 1 (not at all ill) to 7 (extremely ill). Patients that maintained treatment response at Week 3 of the open label treatment phase (N=116) were eligible to be randomized to ongoing treatment with the same dose of lisdexamfetamine dimesylate (N=56) or switched to placebo (N=60) during the double-blind phase. Patients were observed for relapse (treatment failure) during the 6-week double-blind phase. The efficacy endpoint was the proportion of patients with treatment failure during the double-blind phase. Treatment failure was defined as a ≥50% increase (worsening) in the ADHD-RS Total Score and ≥2-point increase in the CGI-S score compared to scores at entry into the double-blind phase. Maintenance of efficacy for patients treated with lisdexamfetamine dimesylate was demonstrated by the significantly lower proportion of patients with treatment failure (9%) compared to patients receiving placebo (75%) at endpoint during the double-blind phase (Study 9, Figure 7).
Table 6: Summary of Primary Efficacy Results from Short-term Studies of Lisdexamfetamine Dimesylate Capsules in Pediatric Patients (Ages 6 to 17) and Adults with ADHD Study Number (Age range) Primary Endpoint Treatment Group Mean Baseline Score (SD) LS Mean Change from Baseline (SE) Placebo-subtracted DifferenceDifference (drug minus placebo) in least-squares mean change from baseline.(95% CI) SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. Study 1
(6 - 12 years)ADHD-RS-IV Lisdexamfetamine Dimesylate Capsules (30 mg/day)Doses statistically significantly superior to placebo. 43.2 (6.7) -21.8 (1.6) -15.6 (-19.9, -11.2) Lisdexamfetamine Dimesylate Capsules (50 mg/day) 43.3 (6.7) -23.4 (1.6) -17.2 (-21.5, -12.9) Lisdexamfetamine Dimesylate Capsules (70 mg/day) 45.1(6.8) -26.7 (1.5) -20.5 (-24.8, -16.2) Placebo 42.4 (7.1) -6.2 (1.6) -- Study 2
(6 - 12 years)Average SKAMP-DS Lisdexamfetamine Dimesylate Capsules (30, 50 or 70 mg/day) --Pre-dose SKAMP-DS was not collected. 0.8 (0.1)LS Mean for SKAMP-DS (Study 2 and 3) or PERMP (Study 8) is post-dose average score over all sessions of the treatment day, rather than change from baseline. -0.9 (-1.1, -0.7) Placebo -- 1.7 (0.1) -- Study 3
(6 - 12 years)Average SKAMP-DS Lisdexamfetamine Dimesylate Capsules (30, 50 or 70 mg/day) 0.9 (1.0)Pre-dose SKAMP-DS (Study 3) or PERMP (Study 8) total score, averaged over both periods. 0.7 (0.1) -0.7 (-0.9, -0.6) Placebo 0.7 (0.9) 1.4 (0.1) -- Study 4
(13 - 17 years)ADHD-RS-IV Lisdexamfetamine Dimesylate Capsules (30 mg/day) 38.3 (6.7) -18.3 (1.2) -5.5 (-9.0, -2.0) Lisdexamfetamine Dimesylate Capsules (50 mg/day) 37.3 (6.3) -21.1 (1.3) -8.3 (-11.8, -4.8) Lisdexamfetamine Dimesylate Capsules (70 mg/day) 37.0 (7.3) -20.7 (1.3) -7.9 (-11.4, -4.5) Placebo 38.5 (7.1) -12.8 (1.2) -- Study 5
(6 - 17 years)ADHD-RS-IV Lisdexamfetamine Dimesylate Capsules (30, 50 or 70 mg/day) 40.7 (7.3) -24.3 (1.2) -18.6 (-21.5, -15.7) Placebo 41.0 (7.1) -5.7 (1.1) -- Study 7
(18 - 55 years)ADHD-RS-IV Lisdexamfetamine Dimesylate Capsules (30 mg/day) 40.5 (6.2) -16.2 (1.1) -8.0 (-11.5, -4.6) Lisdexamfetamine Dimesylate Capsules (50 mg/day) 40.8 (7.3) -17.4 (1.0) -9.2 (-12.6, -5.7) Lisdexamfetamine Dimesylate Capsules (70 mg/day) 41.0 (6.0) -18.6 (1.0) -10.4 (-13.9, -6.9) Placebo 39.4 (6.4) -8.2 (1.4) -- Study 8
(18 - 55 years)Average PERMP Lisdexamfetamine Dimesylate Capsules (30, 50 or 70 mg/day) 260.1 (86.2) 312.9 (8.6) 23.4 (15.6, 31.2) Placebo 261.4 (75.0) 289.5 (8.6) -- Figure 4 LS Mean SKAMP Deportment Subscale Score by Treatment and Time-point for Pediatric Patients Ages 6 to 12 with ADHD after 1 Week of Double Blind Treatment (Study 3)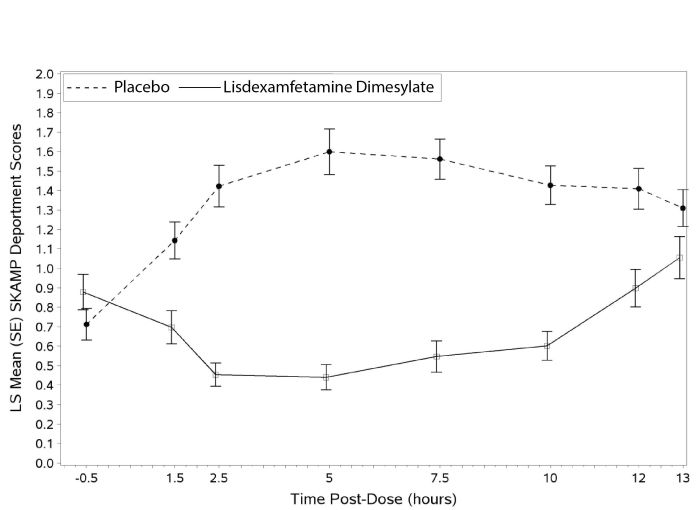
Higher score on the SKAMP-Deportment scale indicates more severe symptoms
Figure 5 Kaplan-Meier Estimated Proportion of Patients with Treatment Failure for Pediatric Patients Ages 6 to 17 (Study 6)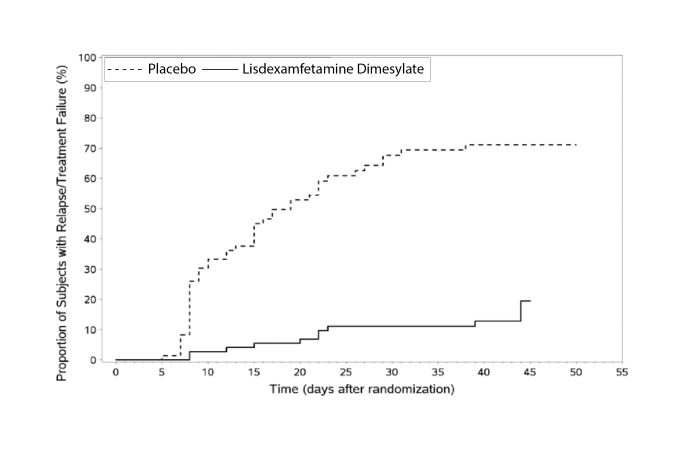 Figure 6 LS Mean (SE) PERMP Total Score by Treatment and Time-point for Adults Ages 18 to 55 with ADHD after 1 Week of Double Blind Treatment (Study 8)
Figure 6 LS Mean (SE) PERMP Total Score by Treatment and Time-point for Adults Ages 18 to 55 with ADHD after 1 Week of Double Blind Treatment (Study 8)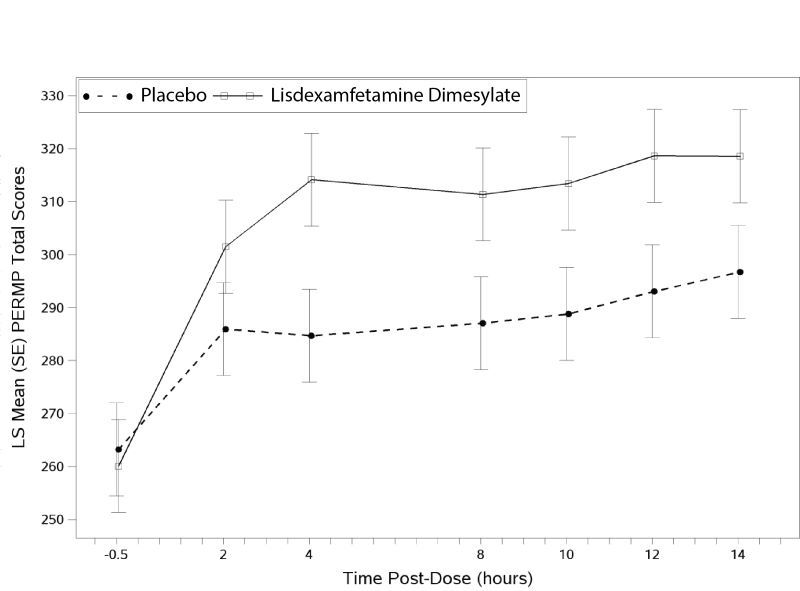
Higher score on the PERMP scale indicates less severe symptoms
Figure 7 Kaplan-Meier Estimated Proportion of Subjects with Relapse in Adults with ADHD (Study 9)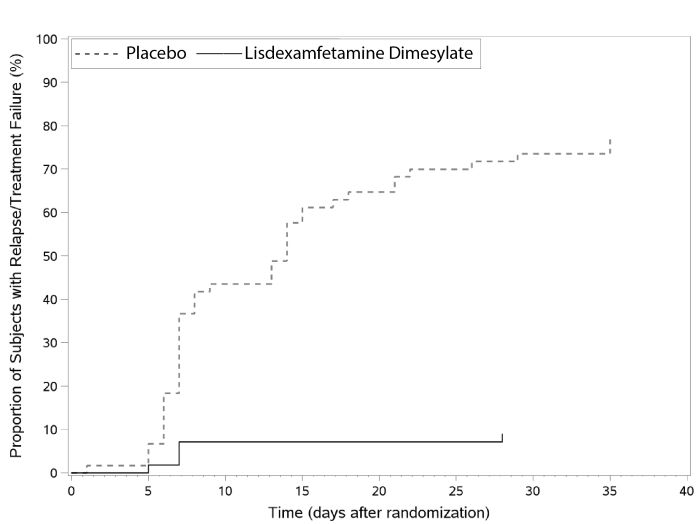
- Moderate to severe binge eating disorder (BED) in adults [see.]
14.2 Binge Eating Disorder (BED)A phase 2 study evaluated the efficacy of lisdexamfetamine dimesylate 30, 50 and 70 mg/day compared to placebo in reducing the number of binge days/week in adults with at least moderate to severe BED. This randomized, double-blind, parallel-group, placebo-controlled, forced-dose titration study (Study 10) consisted of an 11-week double-blind treatment period (3 weeks of forced-dose titration followed by 8 weeks of dose maintenance). Lisdexamfetamine dimesylate 30 mg/day was not statistically different from placebo on the primary endpoint. The 50 and 70 mg/day doses were statistically superior to placebo on the primary endpoint.
The efficacy of lisdexamfetamine dimesylate in the treatment of BED was demonstrated in two 12-week randomized, double-blind, multi-center, parallel-group, placebo-controlled, dose-optimization studies (Study 11 and Study 12) in adults aged 18-55 years (Study 11: N=374, Study 12: N=350) with moderate to severe BED. A diagnosis of BED was confirmed using DSM-IV criteria for BED. Severity of BED was determined based on having at least 3 binge days per week for 2 weeks prior to the baseline visit and on having a Clinical Global Impression Severity (CGI-S) score of ≥4 at the baseline visit. For both studies, a binge day was defined as a day with at least 1 binge episode, as determined from the subject's daily binge diary.
Both 12-week studies consisted of a 4-week dose-optimization period and an 8-week dose-maintenance period. During dose-optimization, subjects assigned to lisdexamfetamine dimesylate began treatment at the titration dose of 30 mg/day and, after 1 week of treatment, were subsequently titrated to 50 mg/day. Additional increases to 70 mg/day were made as tolerated and clinically indicated. Following the dose-optimization period, subjects continued on their optimized dose for the duration of the dose-maintenance period.
The primary efficacy outcome for the two studies was defined as the change from baseline at Week 12 in the number of binge days per week. Baseline is defined as the weekly average of the number of binge days per week for the 14 days prior to the baseline visit. Subjects from both studies on lisdexamfetamine dimesylate had a statistically significantly greater reduction from baseline in mean number of binge days per week at Week 12. In addition, subjects on lisdexamfetamine dimesylate showed greater improvement as compared to placebo across key secondary outcomes with higher proportion of subjects rated improved on the CGI-I rating scale, higher proportion of subjects with 4-week binge cessation, and greater reduction in the Yale-Brown Obsessive Compulsive Scale Modified for Binge Eating (Y-BOCS-BE) total score.
Table 7: Summary of Primary Efficacy Results in BED Study Number Treatment Group Primary Efficacy Measure: Binge Days per Week at Week 12 Mean Baseline Score (SD) LS Mean Change from Baseline (SE) Placebo-subtracted DifferenceDifference (drug minus placebo) in least-squares mean change from baseline.(95% CI) SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. Study 11 Lisdexamfetamine Dimesylate (50 or 70 mg/day)Doses statistically significantly superior to placebo. 4.79 (1.27) -3.87 (0.12) -1.35 (-1.70, -1.01) Placebo 4.60 (1.21) -2.51 (0.13) -- Study 12 Lisdexamfetamine Dimesylate (50 or 70 mg/day) 4.66 (1.27) -3.92 (0.14) -1.66 (-2.04, -1.28) Placebo 4.82 (1.42) -2.26 (0.14) -- A double-blind, placebo controlled, randomized withdrawal design study (Study 13) was conducted to evaluate maintenance of efficacy based on time to relapse between lisdexamfetamine dimesylate and placebo in adults aged 18 to 55 (N=267) with moderate to severe BED. In this longer-term study patients who had responded to lisdexamfetamine dimesylate in the preceding 12-week open-label treatment phase were randomized to continuation of lisdexamfetamine dimesylate or placebo for up to 26 weeks of observation for relapse. Response in the open-label phase was defined as 1 or fewer binge days each week for four consecutive weeks prior to the last visit at the end of the 12-week open-label phase and a CGI-S score of 2 or less at the same visit. Relapse during the double-blind phase was defined as having 2 or more binge days each week for two consecutive weeks (14 days) prior to any visit and having an increase in CGI-S score of 2 or more points compared to the randomized-withdrawal baseline. Maintenance of efficacy for patients who had an initial response during the open-label period and then continued on lisdexamfetamine dimesylate during the 26-week double-blind randomized-withdrawal phase was demonstrated with lisdexamfetamine dimesylate being superior over placebo as measured by time to relapse.
Figure 8 Kaplan-Meier Estimated Proportions of Subjects with Relapse in Adults with BED (Study 13)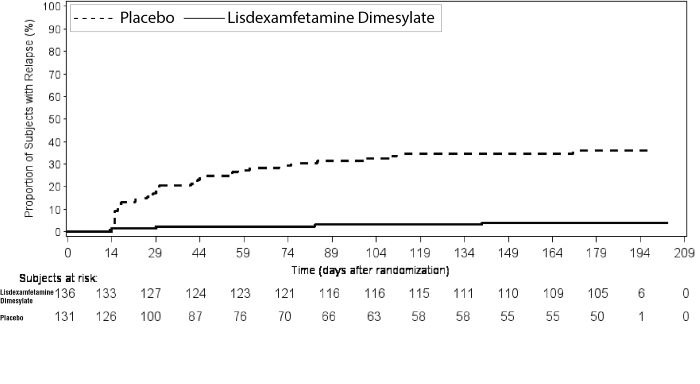
Examination of population subgroups based on age (there were no patients over 65), gender, and race did not reveal any clear evidence of differential responsiveness in the treatment of BED.
| Indicated Population | Initial Dose | Titration Schedule | Recommended Dose | Maximum Dose |
|---|---|---|---|---|
ADHD (Adults and pediatric patients 6 years and older) (Take lisdexamfetamine dimesylate capsules orally in the morning with or without food; avoid afternoon doses because of the potential for insomnia. Lisdexamfetamine dimesylate capsules may be administered in one of the following ways: Information for lisdexamfetamine dimesylate capsules:
Lisdexamfetamine dimesylate capsules can be substituted with VYVANSE chewable tablets on a unit per unit/mg per mg basis (for example, 30 mg capsules for 30 mg chewable tablet) [see Clinical Pharmacology (12.3)] .Do not take anything less than one capsule per day. A single dose should not be divided. | 30 mg every morning | 10 mg or 20 mg weekly | 30 mg to 70 mg per day | 70 mg per day |
BED (Adults) (The recommended starting dosage in adults and pediatric patients 6 years and older is 30 mg once daily in the morning. Dosage may be adjusted in increments of 10 mg or 20 mg at approximately weekly intervals up to maximum recommended dosage of 70 mg once daily [see Clinical Studies (14.1)] . | 30 mg every morning | 20 mg weekly | 50 mg to 70 mg per day | 70 mg per day |
- Prior to treatment, assess for presence of cardiac disease ()
2.4 Dosage for Treatment of Moderate to Severe BED in AdultsThe recommended starting dosage in adults is 30 mg once daily to be titrated in increments of 20 mg at approximately weekly intervals to achieve the recommended target dose of 50 mg to 70 mg once daily. The maximum recommended dosage is 70 mg once daily
[see Clinical Studies (14.2)]. Discontinue lisdexamfetamine dimesylate capsules if binge eating does not improve. - Severe renal impairment: Maximum dose is 50 mg/day ()
2.5 Dosage in Patients with Renal ImpairmentIn patients with severe renal impairment (GFR 15 to < 30 mL/min/1.73 m2), the maximum dosage should not exceed 50 mg once daily. In patients with end stage renal disease (ESRD, GFR < 15 mL/min/1.73 m2), the maximum recommended dosage is 30 mg once daily
[see Use in Specific Populations (8.6)]. - End stage renal disease (ESRD): Maximum dose is 30 mg/day ()
2.5 Dosage in Patients with Renal ImpairmentIn patients with severe renal impairment (GFR 15 to < 30 mL/min/1.73 m2), the maximum dosage should not exceed 50 mg once daily. In patients with end stage renal disease (ESRD, GFR < 15 mL/min/1.73 m2), the maximum recommended dosage is 30 mg once daily
[see Use in Specific Populations (8.6)].
- Capsules: 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, 70 mg ()
3 DOSAGE FORMS AND STRENGTHS- Capsules: 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, 70 mg
Lisdexamfetamine Dimesylate Capsules:- Capsules 10 mg: Size 3 hard gelatin capsule with Opaque pink cap and body (imprinted with E0550 in black ink on cap and 10 mg on body)
- Capsules 20 mg: Size 3 hard gelatin capsule with Opaque Ivory cap and body (imprinted with E0551 in black ink on cap and 20 mg on body)
- Capsules 30 mg: Size 3 hard gelatin capsule with Opaque orange cap and white body (imprinted with E0552 in black ink on cap and 30 mg on body)
- Capsules 40 mg: Size 3 hard gelatin capsule with Opaque blue green cap and white body (imprinted with E0553 in black ink on cap and 40 mg on body)
- Capsules 50 mg: Size 3 hard gelatin capsule with Opaque blue cap and white body (imprinted with E0554 in black ink on cap and 50 mg on body)
- Capsules 60 mg: Size 3 hard gelatin capsule with Opaque Aqua blue cap and body (imprinted with E0555 in black ink on cap and 60 mg on body)
- Capsules 70 mg: Size 3 hard gelatin capsule with Opaque blue cap and orange body (imprinted with E0556 in black ink on cap and 70 mg on body)
- Pregnancy: May cause fetal harm ()
8.1 PregnancyRisk SummaryThe limited available data from published literature and postmarketing reports on use of lisdexamfetamine dimesylate capsules in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage. Adverse pregnancy outcomes, including premature delivery and low birth weight, have been seen in infants born to mothers dependent on amphetamines
[see Clinical Considerations]. In animal reproduction studies, lisdexamfetamine dimesylate (a prodrug of d-amphetamine) had no effects on embryo-fetal morphological development or survival when administered orally to pregnant rats and rabbits throughout the period of organogenesis. Pre- and postnatal studies were not conducted with lisdexamfetamine dimesylate. However, amphetamine (d- to l- ratio of 3:1) administration to pregnant rats during gestation and lactation caused a decrease in pup survival and a decrease in pup body weight that correlated with a delay in developmental landmarks at clinically relevant doses of amphetamine. In addition, adverse effects on reproductive performance were observed in pups whose mothers were treated with amphetamine. Long-term neurochemical and behavioral effects have also been reported in animal developmental studies using clinically relevant doses of amphetamine[see Data].The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical ConsiderationsFetal/Neonatal Adverse ReactionsAmphetamines, such as lisdexamfetamine dimesylate capsules, cause vasoconstriction and thereby may decrease placental perfusion. In addition, amphetamines can stimulate uterine contractions increasing the risk of premature delivery. Infants born to amphetamine-dependent mothers have an increased risk of premature delivery and low birth weight.
Monitor infants born to mothers taking amphetamines for symptoms of withdrawal such as feeding difficulties, irritability, agitation, and excessive drowsiness.
DataAnimal DataLisdexamfetamine dimesylate had no apparent effects on embryo-fetal morphological development or survival when administered orally to pregnant rats and rabbits throughout the period of organogenesis at doses of up to 40 and 120 mg/kg/day, respectively. These doses are approximately 5.5 and 33 times, respectively, the maximum recommended human dose (MRHD) of 70 mg/day given to adults, on a mg/m2body surface area basis.
A study was conducted with amphetamine (d- to l- enantiomer ratio of 3:1) in which pregnant rats received daily oral doses of 2, 6, and 10 mg/kg from gestation day 6 to lactation day 20. All doses caused hyperactivity and decreased weight gain in the dams. A decrease in pup survival was seen at all doses. A decrease in pup body weight was seen at 6 and 10 mg/kg which correlated with delays in developmental landmarks, such as preputial separation and vaginal opening. Increased pup locomotor activity was seen at 10 mg/kg on day 22 postpartum but not at 5 weeks postweaning. When pups were tested for reproductive performance at maturation, gestational weight gain, number of implantations, and number of delivered pups were decreased in the group whose mothers had been given 10 mg/kg.
A number of studies from the literature in rodents indicate that prenatal or early postnatal exposure to amphetamine (d- or d, l-) at doses similar to those used clinically can result in long-term neurochemical and behavioral alterations. Reported behavioral effects include learning and memory deficits, altered locomotor activity, and changes in sexual function.
- Lactation:Breastfeeding not recommended ()
8.2 LactationRisk SummaryLisdexamfetamine is a pro-drug of dextroamphetamine. Based on limited case reports in published literature, amphetamine (d-or d, l-) is present in human milk, at relative infant doses of 2% to 13.8% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.9 and 7.5. There are no reports of adverse effects on the breastfed infant. Long-term neurodevelopmental effects on infants from amphetamine exposure are unknown. It is possible that large dosages of dextroamphetamine might interfere with milk production, especially in women whose lactation is not well established. Because of the potential for serious adverse reactions in nursing infants, including serious cardiovascular reactions, blood pressure and heart rate increase, suppression of growth, and peripheral vasculopathy, advise patients that breastfeeding is not recommended during treatment with lisdexamfetamine dimesylate capsules.