Methocarbamol Tablets, Usp, 500 Mg
(Methocarbamol)Methocarbamol Tablets, Usp, 500 Mg Prescribing Information
Methocarbamol Tablets, USP are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of methocarbamol has not been clearly identified, but may be related to its sedative properties. Methocarbamol does not directly relax tense skeletal muscles in man.
Methocarbamol Tablets, USP, 500 mg – Adults:
Initial dosage: 3 tablets four times daily
Maintenance dosage: 2 tablets four times daily
Methocarbamol Tablets, USP, 750 mg – Adults:
Initial dosage: 2 tablets four times daily
Maintenance dosage: 1 tablet every 4 hours or 2 tablets three times daily
Six grams a day are recommended for the first 48 to 72 hours of treatment. (For severe conditions 8 grams a day may be administered.) Thereafter, the dosage can usually be reduced to approximately 4 grams a day.
Methocarbamol Tablets, USP are contraindicated in patients hypersensitive to methocarbamol or to any of the tablet components.
Adverse reactions reported coincident with the administration of methocarbamol include:
See
Since methocarbamol may possess a general CNS depressant effect, patients receiving Methocarbamol Tablets, USP should be cautioned about combined effects with alcohol and other CNS depressants.
Safe use of Methocarbamol Tablets, USP has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following
Methocarbamol may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. Patients should be cautioned about operating machinery, including automobiles, until they are reasonably certain that methocarbamol therapy does not adversely affect their ability to engage in such activities.
Patients should be cautioned that methocarbamol may cause drowsiness or dizziness, which may impair their ability to operate motor vehicles or machinery.
Because methocarbamol may possess a general CNS-depressant effect, patients should be cautioned about combined effects with alcohol and other CNS depressants.
See
Methocarbamol may inhibit the effect of pyridostigmine bromide. Therefore, methocarbamol should be used with caution in patients with myasthenia gravis receiving anticholinesterase agents.
Methocarbamol may cause a color interference in certain screening tests for 5-hydroxyindoleacetic acid (5-HIAA) using nitrosonaphthol reagent and in screening tests for urinary vanillylmandelic acid (VMA) using the Gitlow method.
Long-term studies to evaluate the carcinogenic potential of methocarbamol have not been performed. No studies have been conducted to assess the effect of methocarbamol on mutagenesis or its potential to impair fertility.
Animal reproduction studies have not been conducted with methocarbamol. It is also not known whether methocarbamol can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methocarbamol Tablets, USP should be given to a pregnant woman only if clearly needed.
Safe use of Methocarbamol Tablets, USP has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following
Methocarbamol and/or its metabolites are excreted in the milk of dogs; however, it is not known whether methocarbamol or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Methocarbamol Tablets, USP are administered to a nursing woman.
Safety and effectiveness of Methocarbamol Tablets, USP in pediatric patients below the age of 16 have not been established.
Methocarbamol may inhibit the effect of pyridostigmine bromide. Therefore, methocarbamol should be used with caution in patients with myasthenia gravis receiving anticholinesterase agents.
Methocarbamol Tablets, USP, a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties.
The chemical name of methocarbamol is 3-(2-methoxyphenoxy)-1,2-propanediol 1-carbamate and has the empirical formula C11H15NO5. Its molecular weight is 241.24. The structural formula is shown below.
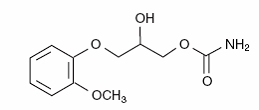
Methocarbamol is a white powder, sparingly soluble in water and chloroform, soluble in alcohol (only with heating) and propylene glycol, and insoluble in benzene and
Methocarbamol Tablets, USP 500 mg are available as a white, round, scored, film-coated tablet containing 500 mg of methocarbamol, USP for oral administration. The inactive ingredients present are microcrystalline cellulose, croscarmellose sodium, povidone (k-30), sodium lauryl sulfate, colloidal silicon dioxide, magnesium stearate, hypromellose, titanium dioxide, polyethylene glycol, polysorbate 80.
Methocarbamol Tablets, USP 750 mg are available as a white, capsule-shaped, film-coated tablet containing 750 mg of methocarbamol, USP for oral administration. The inactive ingredients present are microcrystalline cellulose, croscarmellose sodium, povidone (k-30), sodium lauryl sulfate, colloidal silicon dioxide, magnesium stearate, hypromellose, titanium dioxide, polyethylene glycol, polysorbate 80.