Phenoxybenzamine Hydrochloride
Phenoxybenzamine Hydrochloride Prescribing Information
Phenoxybenzamine Hydrochloride Capsules are indicated in the treatment of pheochromocytoma, to control episodes of hypertension and sweating. If tachycardia is excessive, it may be necessary to use a
The dosage should be adjusted to fit the needs of each patient. Small initial doses should be slowly increased until the desired effect is obtained or the side effects from blockade become troublesome. After each increase, the patient should be observed on that level before instituting another increase. The dosage should be carried to a point where symptomatic relief and/or objective improvement are obtained, but not so high that the side effects from blockade become troublesome.
Initially, 10 mg of phenoxybenzamine hydrochloride twice a day. Dosage should be increased every other day, usually to 20 to 40 mg 2 or 3 times a day, until an optimal dosage is obtained, as judged by blood pressure control.
Long-term use of phenoxybenzamine is not recommended (see PRECAUTIONS Carcinogenesis and Mutagenesis).
Conditions where a fall in blood pressure may be undesirable; hypersensitivity to the drug or any of its components.
The following adverse reactions have been observed, but there are insufficient data to support an estimate of their frequency.
Autonomic Nervous System*: Postural hypotension, tachycardia, inhibition of ejaculation, nasal congestion, miosis.
*These so-called "side effects" are actually evidence of adrenergic blockade and vary according to the degree of blockade.
Miscellaneous: Gastrointestinal irritation, drowsiness, fatigue.
Phenoxybenzamine hydrochloride may interact with compounds that stimulate both
Phenoxybenzamine hydrochloride blocks hyperthermia production by levarterenol, and blocks hypothermia production by reserpine.
Each Phenoxybenzamine Hydrochloride Capsule, USP 10 mg is a size #3 capsule with red transparent cap and body, imprinted “365” on cap and “novitium 10 mg” on body with grey ink, filled with white to off white powder containing 10 mg of Phenoxybenzamine Hydrochloride USP and the following inactive ingredients: lactose and talc.
Each of the empty hard gelatin capsule contains gelatin, purified water, sodium lauryl sulfate, D&C Red 33, FD&C Yellow 6 and FD&C Red 3.
The imprinting ink contains the following: shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, potassium hydroxide, titanium dioxide, black iron oxide and purified water.
Phenoxybenzamine Hydrochloride is N-(2-Chloroethyl)-N-(1-methyl-2-phenoxyethyl) benzylamine hydrochloride:
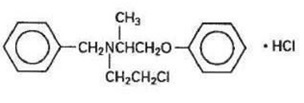
Phenoxybenzamine hydrochloride is a white to off-white powder with a molecular weight of 340.3, which melts between 136° and 141°C. It is soluble in water, alcohol and chloroform; insoluble in ether.