Sucralfate
(Sucralfate Oral)Sucralfate Prescribing Information
Sucralfate Oral Suspension is indicated in the short-term (up to 8 weeks) treatment of active duodenal ulcer.
Antacids may be prescribed as needed for relief of pain but should not be taken within one-half hour before or after Sucralfate Oral Suspension.
While healing with sucralfate may occur during the first week or two, treatment should be continued for 4 to 8 weeks unless healing has been demonstrated by x-ray or endoscopic examination.
Clinical studies of Sucralfate Oral Suspension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function (see
Patients
Call your doctor for medical advice about side effects. You may report side effects to Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Sucralfate Oral Suspension is contraindicated for patients with known hypersensitivity reactions to the active substance or to any of the excipients.
Adverse reactions to sucralfate tablets in clinical trials were minor and only rarely led to discontinuation of the drug. In studies involving over 2700 patients treated with sucralfate, adverse effects were reported in 129 (4.7%).
Constipation was the most frequent complaint (2%). Other adverse effects reported in less than 0.5% of the patients are listed below by body system:
Cases of bronchospasm, laryngeal edema and respiratory tract edema have been reported with an unknown oral formulation of sucralfate.
Cases of hyperglycemia have been reported with sucralfate.
Bezoars have been reported in patients treated with sucralfate. The majority of patients had underlying medical conditions that may predispose to bezoar formation (such as delayed gastric emptying) or were receiving concomitant enteral tube feedings.
Some studies have shown that simultaneous sucralfate administration in healthy volunteers reduced the extent of absorption (bioavailability) of single doses of the following: cimetidine, digoxin, fluoroquinolone antibiotics, ketoconazole, l-thyroxine, phenytoin, quinidine, ranitidine, tetracycline, and theophylline. Subtherapeutic prothrombin times with concomitant warfarin and sucralfate therapy have been reported in spontaneous and published case reports. However, two clinical studies have demonstrated no change in either serum warfarin concentration or prothrombin time with the addition of sucralfate to chronic warfarin therapy.
The mechanism of these interactions appears to be nonsystemic in nature, presumably resulting from sucralfate binding to the concomitant agent in the gastrointestinal tract. In all cases studied to date (cimetidine, ciprofloxacin, digoxin, norfloxacin, ofloxacin, and ranitidine), dosing the concomitant medication 2 hours before sucralfate eliminated the interaction. Due to Sucralfate Oral Suspension's potential to alter the absorption of some drugs, Sucralfate Oral Suspension should be administered separately from other drugs when alterations in bioavailability are felt to be critical. In these cases, patients should be monitored appropriately.
Sucralfate Oral Suspension contains sucralfate, USP and sucralfate is an α-D-glucopyranoside, β-D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex.
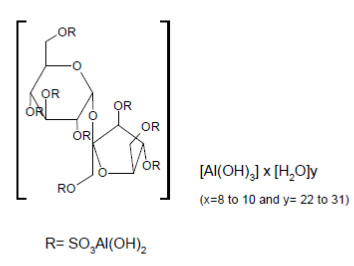
Sucralfate Oral Suspension for oral administration contains 1 g of sucralfate, USP per 10 mL.
Sucralfate Oral Suspension also contains: cherry flavor, colloidal silicon dioxide, FD&C Red #40, glycerin, methylcellulose, methylparaben, microcrystalline cellulose, purified water, simethicone emulsion, and sorbitol solution. Therapeutic category: antiulcer.